Heat Integrated Distillation Operation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Extraction of Wax from Sorghum Bran •
i EXTRACTION OF WAX FROM SORGHUM BRAN • 1 HSIEN-WEN HSU <& . ***£/ B. S., National Taiwan University, China, 1951 A THESIS » submitted in partial fulfillment of the requirements for the degree MASTER OF SCIENCE - Department of Chemical Engineering KANSAS STATE COLLEGE OF AGRICULTURE AND APPLIED SCIENCE - 1955 » k Lo i~i ii Q^cu^v^Js TABLE OF CONTENTS INTRODUCTION 1 PREVIOUS WORK 3 MATERIALS 6 GLASS SOXHLET EXTRACTION AND ANALYSIS OF WAX AND OIL IN MISCELLA 7 Procedure of Using Soxhlet Extractor 7 Separation of Wax from Oil by the Acetone Method.. 8 Summary of Results 9 PILOT PLANT EXTRACTION 15 SEPARATION OF WAX AND OIL 16 Effect of Temperature in the Acetone Method 16 Urea Complex Method of Separation. 17 Theory 17 Experimental Procedure 22 Summary of Results 26 COST ESTIMATION OF A WAX RECOVERY PLANT 29 DISCUSSION 30 CONCLUSIONS 35 ACKNOWLEDGMENTS 36 BIBLIOGRAPHY 37 APPENDIX 39 INTRODUCTION i One of the functions of the Agricultural Experiment Station is the never ending search for means of better and fuller utilization of the agri- cultural products that are of particular interest in the state of Kansas. In line with this broad objective, the extraction of economically valuable lipid materials from the sorghum bran has been studied in several theses in the Departments of Chemical Engineering and Chemistry in recent years. Generally the emphasis has b. en placed upon either the chemical and physical properties of these lipides or the equipment design and performance for the solvent ex- traction operation. A brief review of the previous work is presented in the ensuing section. -

The Separation of Three Azeotropes by Extractive Distillation by An-I Yeh A
The separation of three azeotropes by extractive distillation by An-I Yeh A thesis submitted in partial fulfillment of the requirement for the degree of Master of Science in Chemical Engineering Montana State University © Copyright by An-I Yeh (1983) Abstract: Several different kinds of extractive distillation agents were investigated to affect the separation of three binary liquid mixtures, isopropyl ether - acetone, methyl acetate - methanol, and isopropyl ether - methyl ethyl ketone. Because of the small size of the extractive distillation column, relative volatilities were assumed constant and the Fenske equation was used to calculate the relative volatilities and the number of minimum theoretical plates. Dimethyl sulfoxide was found to be a good extractive distillation agent. Extractive distillation when employing a proper agent not only negated the azeotropes of the above mixtures, but also improved the efficiency of separation. This process could reverse the relative volatility of isopropyl ether and acetone. This reversion was also found in the system of methyl acetate and methanol when nitrobenzene was the agent. However, normal distillation curves were obtained for the system of isopropyl ether and methyl ethyl ketone undergoing extractive distillation. In the system of methyl acetate and methanol, the relative volatility decreased as the agents' carbon number increased when glycols were used as the agents. In addition, the oxygen number and the locations of hydroxyl groups in the glycols used were believed to affect the values of relative volatility. An appreciable amount of agent must be maintained in the column to affect separation. When dimethyl sulfoxide was an agent for the three systems studied, the relative volatility increased as the addition rate increased. -

Innovation in Continuous Rectification for Tequila Production
processes Communication Innovation in Continuous Rectification for Tequila Production Estarrón-Espinosa Mirna, Ruperto-Pérez Mariela, Padilla-de la Rosa José Daniel * and Prado-Ramírez Rogelio * Centro de Investigación y Asistencia en Tecnología y Diseño del Estado de Jalisco (CIATEJ), Av. Normalistas No. 800, C.P. 44720 Guadalajara, Jalisco, Mexico; [email protected] (E.-E.M.); [email protected] (R.-P.M.) * Correspondence: [email protected] (P.-d.l.R.J.D.); [email protected] (P.-R.R.); Tel.: +33-33455200 (P.-d.l.R.J.D.) Received: 23 March 2019; Accepted: 6 May 2019; Published: 14 May 2019 Abstract: In this study, a new process of continuous horizontal distillation at a pilot level is presented. It was applied for the first time to the rectification of an ordinario fraction obtained industrially. Continuous horizontal distillation is a new process whose design combines the benefits of both distillation columns, in terms of productivity and energy savings (50%), and distillation stills in batch, in terms of the aromatic complexity of the distillate obtained. The horizontal process of continuous distillation was carried out at the pilot level in a manual mode, obtaining five accumulated fractions of distillate that were characterized by gas chromatography (GC-FID). The tequila obtained from the rectification process in this new continuous horizontal distillation process complies with the content of methanol and higher alcohols regulated by the Official Mexican Standard (NOM-006-SCFI-2012). Continuous horizontal distillation of tequila has potential energy savings of 50% compared to the traditional process, besides allowing products with major volatile profiles within the maximum limits established by the regulation for this beverage to be obtained. -

Page2 Sidebar 1.Pdf
Headquarters & Forge Americas Office [email protected] [email protected] +49 (0) 7161 / 97830 215.242.6806 +49 (0) 7161 / 978321 fax fax 215.701.9725 artisan distilling systems 600 liter whiskey still the fine art of distillery technology Germany’s oldest distillery fabricator, since 1869, combining traditional family craftsmanship with leading eau-de-vie distillery innovations and technologies. Meticulously custom-crafted artisan copper pot still systems for all the great distilling traditions, and efficient continuous plants, grappa distillery in copper and stainless steel, for all capacities and applications: 450 liter artisan pot stills – brandy & vodka vodka, whiskey, eaux-de-vie, brandy, rum, gin, grappa, tequila, aguardientes… 1000 liter artisan vodka system 650 liter system with CADi automation continuous mash stripping column C. CARL Ziegelstraße 21 Americas Office Brewing & Distilling Ing. GmbH D-73033 Göppingen PO Box 4388 Technologies Corp. www.christiancarl.com Germany Philadelphia, PA 19118-8388 www.brewing-distilling.com CARL artisan distillery systems the fine art of distillery technology CARL custom-builds each distillery to order in our family shop near Stuttgart in Swabia, with the attention and care of crafting a finely- tuned instrument. All-the-while, we stay focused on the continued development of our distillery technology. There are always new ideas and realizations, such our the in-house developed CARL CADi distillery automation or our patented aroma bubble plate technologies, Our innovations have fostered CARL’s nearly 140 years of family tradition and experience as Germany’s oldest and most respected distillery fabricator, with thousands of successful commissions worldwide. form and function Our diverse customers, from small farmers to winemakers to brewers to large spirits houses, show great enthusiasm and appreciation for the aesthetics and functionality of a CARL distillery: its design, its form, classic and intuitively easy to understand, clear in conception. -

Distillation 65 Chem 355 Jasperse DISTILLATION
Distillation 65 Chem 355 Jasperse DISTILLATION Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize. The vapors produced are subsequently passed through a water-cooled condenser. Upon cooling, the vapor returns to it’s liquid phase. The liquid can then be collected. The ability to separate mixtures of liquids depends on differences in volatility (the ability to vaporize). For separation to occur, the vapor that is condensed and collected must be more pure than the original liquid mix. Distillation can be used to remove a volatile solvent from a nonvolatile product; to separate a volatile product from nonvolatile impurities; or to separate two or more volatile products that have sufficiently different boiling points. Vaporization and Boiling When a liquid is placed in a closed container, some of the molecules evaporate into any unoccupied space in the container. Evaporation, which occurs at temperatures below the boiling point of a compound, involves the transition from liquid to vapor of only those molecules at the liquid surface. Evaporation continues until an equilibrium is reached between molecules entering and leaving the liquid and vapor states. The pressure exerted by these gaseous molecules on the walls of the container is the equilibrium vapor pressure. The magnitude of this vapor pressure depends on the physical characteristics of the compound and increases as temperature increases. In an open container, equilibrium is never established, the vapor can simply leave, and the liquid eventually disappears. But whether in an open or closed situation, evaporation occurs only from the surface of the liquid. -

Distillation 6
CHAPTER Energy Considerations in Distillation 6 Megan Jobson School of Chemical Engineering and Analytical Science, The University of Manchester, Manchester, UK CHAPTER OUTLINE 6.1 Introduction to energy efficiency ....................................................................... 226 6.1.1 Energy efficiency: technical issues................................................... 227 6.1.1.1 Heating.................................................................................... 227 6.1.1.2 Coolingdabove ambient temperatures...................................... 229 6.1.1.3 Coolingdbelow ambient temperatures ...................................... 230 6.1.1.4 Mechanical or electrical power.................................................. 232 6.1.1.5 Summarydtechnical aspects of energy efficiency ..................... 233 6.1.2 Energy efficiency: process economics............................................... 233 6.1.2.1 Heating.................................................................................... 233 6.1.2.2 Cooling..................................................................................... 235 6.1.2.3 Summarydprocess economics and energy efficiency ............... 237 6.1.3 Energy efficiency: sustainable industrial development........................ 237 6.2 Energy-efficient distillation ............................................................................... 237 6.2.1 Energy-efficient distillation: conceptual design of simple columns...... 238 6.2.1.1 Degrees of freedom in design .................................................. -

Modelling of Crude Oil Distillation
Modelling of Crude Oil Distillation Modellering av råoljedestillation JENN Y BEATRIC E SOUC K K T H C h em i ca l S ci en c e and E n gi n e e r i n g Master’s Thesis in Chemical Engineering Stockholm, Sweden 2012 " One never notices what has been done; one can only see what remains to be done" (Marie Curie, 1867-1934) 1 Sammanfattning Under de föhållanden som reservoarens miljö erbjuder, definieras en petroleumvätska av dess termodynamiska och volymetriska egenskaper och av dess fysikalisk-kemiska egenskaper. För att korrekt simulera bearbetningen av dessa vätskor under produktion, deras beteende modelleras från experimentella data Med tillkomsten av nya regler och oflexibilitet som finns på tullbestämmelser vid gränserna idag, har forskningscenter stora svårigheter att få större mängder prover levererade. Av den anledningen, trots att det finns flera metoder för att karakterisera de olika komponenterna av råolja, tvingas laboratorier att vända sig mer och mer till alternativa analysmetoder som kräver mindre provvolymer: mikrodestillation, gaskromatografi, etc. Mikrodestillation, som är en snabb och helt datoriserad teknik, visar sig kunna ersätta standarddestillation för analys av flytande petroleumprodukter. Fördelar med metoden jämfört med standarddestillering är minskad arbetstidsåtgång med minst en faktor 4. Därtill krävs endast en [24] begränsad provvolym (några mikroliter) i jämförelse med standarddestillation. Denna rapport syftar till att skapa en enkel modell som kan förutsäga avkastningskurvan av fysisk destillation, utan att använda mikrodestillationsteknik. De resultat som erhölls genom gaskromatografiska analyser möjliggjorde modelleringen av det vätskebeteendet hos det analyserade provet. Efter att ha identifierat och behandlat praktiskt taget alla viktiga aspekter av mikro destillation genom simuleringar med PRO/II, fann jag att, oberoende av inställningen och den termodynamiska metod som används, det alltid finns stora skillnader mellan simulering och mikro destillation. -
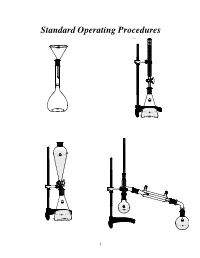
Standard Operating Procedures
Standard Operating Procedures 1 Standard Operating Procedures OVERVIEW In the following laboratory exercises you will be introduced to some of the glassware and tech- niques used by chemists to isolate components from natural or synthetic mixtures and to purify the individual compounds and characterize them by determining some of their physical proper- ties. While working collaboratively with your group members you will become acquainted with: a) Volumetric glassware b) Liquid-liquid extraction apparatus c) Distillation apparatus OBJECTIVES After finishing these sessions and reporting your results to your mentor, you should be able to: • Prepare solutions of exact concentrations • Separate liquid-liquid mixtures • Purify compounds by recrystallization • Separate mixtures by simple and fractional distillation 2 EXPERIMENT 1 Glassware Calibration, Primary and Secondary Standards, and Manual Titrations PART 1. Volumetric Glassware Calibration Volumetric glassware is used to either contain or deliver liquids at a specified temperature. Glassware manufacturers indicate this by inscribing on the volumetric ware the initials TC (to contain) or TD (to deliver) along with the calibration temperature, which is usually 20°C1. Volumetric glassware must be scrupulously clean before use. The presence of streaks or droplets is an indication of the presence of a grease film. To eliminate grease from glassware, scrub with detergent solution, rinse with tap water, and finally rinse with a small portion of distilled water. Volumetric flasks (TC) A volumetric flask has a large round bottom with only one graduation mark positioned on the long narrow neck. Graduation Mark Stopper The position of the mark facilitates the accurate and precise reading of the meniscus. If the flask is used to prepare a solution starting with a solid compound, add small amounts of sol- vent until the entire solid dissolves. -

Review of Extractive Distillation. Process Design, Operation
Review of Extractive Distillation. Process design, operation optimization and control Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, Xinqiang You To cite this version: Vincent Gerbaud, Ivonne Rodríguez-Donis, Laszlo Hegely, Péter Láng, Ferenc Dénes, et al.. Review of Extractive Distillation. Process design, operation optimization and control. Chemical Engineering Research and Design, Elsevier, 2019, 141, pp.229-271. 10.1016/j.cherd.2018.09.020. hal-02161920 HAL Id: hal-02161920 https://hal.archives-ouvertes.fr/hal-02161920 Submitted on 21 Jun 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Open Archive Toulouse Archive Ouverte OATAO is an open access repository that collects the work of Toulouse researchers and makes it freely available over the web where possible This is an author’s version published in: http://oatao.univ-toulouse.fr/239894 Official URL: https://doi.org/10.1016/j.cherd.2018.09.020 To cite this version: Gerbaud, Vincent and Rodríguez-Donis, Ivonne and Hegely, Laszlo and Láng, Péter and Dénes, Ferenc and You, Xinqiang Review of Extractive Distillation. Process design, operation optimization and control. (2018) Chemical Engineering Research and Design, 141. -

Computer Simulation of a Multicomponent, Multistage Batch Distillation Process
COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS By BRUCE EARL BAUGHER /J Bachelor of Science in Chemical Engineering Oklahoma State University Stillwater, Oklahoma 1985 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of MASTER OF SCIENCE July, 1988 \\,~s~ ~ \'\~~ B~~~to' <:.o?. ·;)._, ~ r COMPUTER SIMULATION OF A MULTICOMPONENT, MULTISTAGE BATCH DISTILLATION PROCESS Thesis Approved: Thesis Advisor U.bJ ~~- ~MJYl~ Dean of the GradU~te College ABSTRACT The material and energy balance equations for a batch distillation column were derived and a computer simulation was developed to solve these equations. The solution follows a modified Newton-Raphson approach for inverting and solving the matrices of material and energy balance equations. The model is unique in that it has been designed to handle hypothetical hydrocarbon components. The simulation can handle columns up to 50 trays and systems of up to 100 components. The model has been used to simulate True Boiling Point (TBP) diagrams for a variety of crude oils. This simulation is also applicable to simple laboratory batch distillations. The model was designed to accurately combine data files to simulate actual crude blending procedures. The model will combine files and calculate the results quickly and easily. The simulation involves removing material from the column at steady-state. The removal fraction is made small enough to approach continuity. This simulation can be adapted for use on microcomputers, although it will require extensive computation time. ACKNOWLEDGMENTS It has been a great pleasure to work with my thesis advisor, Dr. -

Laboratory Rectifying Stills of Glass 2
» LABORATORY RECTIFYING STILLS OF GLASS 2 By Johannes H. Bruun 3 and Sylvester T. Schicktanz 3 ABSTRACT A complete description is given of a set of all-glass rectifying stills, suitable for distillation at pressures ranging from atmospheric down to about 50 mm. The stills are provided with efficient bubbling-cap columns containing 30 to 60 plates. Adiabatic conditions around the column are maintained by surrounding it with a jacket provided with a series of independent electrical heating units. Suitable means are provided for adjusting or maintaining the reflux ratio at the top of the column. For the purpose of conveniently obtaining an accurate value of the true boiling points of the distillates a continuous boiling-point apparatus is incor- porated in the receiving system. An efficient still of the packed-column type for distillations under pressures less than 50 mm is also described. Methods of operation and efficiency tests are given for the stills. CONTENTS Page I. Introduction 852 II. General features of the still assembly 852 III. Still pot 853 IV. Filling tube and heater for the still pot 853 V. Rectifying column 856 1. General features 856 2. Large-size column 856 3. Medium-size column 858 4. Small-size column 858 VI. Column jacket 861 VII. Reflux regulator 861 1. With variable reflux ratio 861 2. With constant reflux ratio 863 VIII. The continuous boiling-point apparatus 863 IX. Condensers and receiver 867 X. The mounting of the still 867 XI. Manufacture and transportation of the bubbling-cap still 870 XII. Operation of the bubbling-cap still 870 XIII. -

Distillation Handbook
I distillation terminology To provide a better understanding of the distillation process, the following briefly explains the terminology most often encountered. SOLVENT RECOVERY The term "solvent recovery" often has been a somewhat vague label applied to the many and very different ways in which solvents can be reclaimed by industry. One approach employed in the printing and coatings industries is merely to take impure solvents containing both soluble and insoluble particles and evapo- rate the solvent from the solids. For a duty of this type, APV offers the Paraflash evaporator, a compact unit which combines a Paraflow plate heat exchanger and a small separator. As the solvent ladened liquid is recirculated through the heat exchanger, it is evaporated and the vapor and liquid separated. This will recover a solvent but it will not separate solvents if two or more are present. Afurther technique is available to handle an air stream that carries solvents. By chilling the air by means of vent condensers or refrigeration equipment, the solvents can be removed from the condenser. Solvents also can be recovered by using extraction, adsorption, absorption, and distillation methods. S 0 LV E NT EXT R ACT I0 N Essentially a liquid/liquid process where one liquid is used to extract another from a secondary stream, solvent extraction generally is performed in a column somewhat similar to a normal distillation column. The primary difference is that the process involves two liquids instead of liquid and vapor. During the process, the lighter (i.e., less dense) liquid is charged to the base of the column and rises through packing or trays while the more dense liquid descends.