Information Sheet Etizolam Version: 1.0 Original Version: 17/06/2014
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Socio-Demographic and Clinical Characteristics of Benzodiazepine Long-Term Users: Results from a Tertiary Care Center ⁎ F
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Florence Research Available online at www.sciencedirect.com ScienceDirect Comprehensive Psychiatry 69 (2016) 211–215 www.elsevier.com/locate/comppsych Socio-demographic and clinical characteristics of benzodiazepine long-term users: Results from a tertiary care center ⁎ F. Coscia, , G. Mansuetoa, M. Faccinib, R. Casarib, F. Lugobonib aDepartment of Health Sciences, University of Florence, via di San Salvi 12, 50135, Florence, Italy bAddiction Unit, Verona University Hospital, piazzale Aristide Stefani 1, 37126, Verona, Italy Abstract Objective: The use of benzodiazepines (BDZs) represents a critical issue since a long-term treatment may lead to dependence. This study aimed at evaluating socio-demographic and clinical characteristics of BZD long-term users who followed a detoxification program at a tertiary care center. Method: Two hundred-five inpatients were evaluated. Socio-demographic (e.g., gender, age, education) and clinical information (e.g., BZD used, dose, reason of prescription) was collected. BZDs dose was standardized as diazepam dose equivalents and was compared via the Defined Daily Dose (DDD). Chi-square, Fisher test, ANOVA and Bonferroni analyses were performed. Results: Females were more frequently BDZ long-term users than males. Hypnotic BZDs were frequently prescribed for problems different from sleep disturbances. Lorazepam, alprazolam, and lormetazepam were the most prescribed drugs. Lorazepam was more frequently used by males, consumed for a long period, in pills, and prescribed for anxiety. Lormetazepam was more frequently consumed by females with a high school education, having a psychiatric disorder, taken in drops and prescribed for insomnia. -

ETIZOLAM Critical Review Report Agenda Item 4.13
ETIZOLAM Critical Review Report Agenda Item 4.13 Expert Committee on Drug Dependence Thirty-ninth Meeting Geneva, 6-10 November 2017 39th ECDD (2017) Agenda item 4.13 Etizolam Page 2 of 20 39th ECDD (2017) Agenda item 4.13 Etizolam Contents Acknowledgements.................................................................................................................................. 5 Summary...................................................................................................................................................... 6 1. Substance identification ....................................................................................................................... 7 A. International Nonproprietary Name (INN).......................................................................................................... 7 B. Chemical Abstract Service (CAS) Registry Number .......................................................................................... 7 C. Other Chemical Names ................................................................................................................................................... 7 D. Trade Names ....................................................................................................................................................................... 7 E. Street Names ....................................................................................................................................................................... 8 F. Physical Appearance -

ETIZOLAM in POST-MORTEM CASES Joanna Hockenhull1
ETIZOLAM IN POST-MORTEM CASES Joanna Hockenhull1 1Toxicology Unit, Faculty of Medicine, Imperial College London. INTRODUCTION: Etizolam [4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepine] is a benzodiazepine analogue. The benzene ring is replaced with a thiophene ring making the drug a ‘thienodiazepine’. This poster reviews four post-mortem cases, submitted to Imperial College Toxicology Unit, in which etizolam was detected in the post-mortem, femoral blood. RESULTS: The findings are summarised in the tables below. Medicinal use: • Widely available in Japan (Depas, Sedekopan ) and India (Etilaam, Etizola). CASE 1: CASE 2: • Adult daily doses range from 0.5 – 3.0 milligrams. • 52 year old female • 47 year old male • Used in short-term treatment of insomnia or anxiety. 1 • Found dead in church yard surrounded by empty • History of alcohol abuse and depression 2 • Acts as a full agonist at the benzodiazepine receptor. medication packets • Has potent hypnotic properties comparable with other short-acting • Found dead in bed surrounded by empty medication benzodiazepines. Drug Blood Concentration packets. • (Etizolam has anxiolytic effects six times greater than diazepam). 3 ETIZOLAM 0.09 µg/ml Drug Blood Concentration 4 • Thought to have reduced tolerance/dependence than classical benzodiazepines. Diphenhydramine High therapeutic ETIZOLAM 0.46 µg/ml FIGURE 1: Structure of Etizolam • However, long-term use may produce similar side-effects to benzodiazepines: Venlafaxine 7.10 µg/ml 5 Amitriptyline High therapeutic addiction, hostile behaviour, memory loss and severe withdrawal. Mirtazapine 1.41 µg/ml Illicit use: Ethanol <10 mg/dL Citalopram 0.79 µg/ml • Etizolam is not licenced as a medicine in the UK. -

CREW NPS Booklet
NEW Psychoactive DRUGS V1.7 05/15 Service availability Drop-in: Monday – Wednesday: 1pm – 5pm, Thursday: 3pm – 7pm, Friday – Saturday: 1pm – 5pm, Sunday: Closed Telephone information and support: Monday – Friday: 10am – 5pm Online information and chatroom support: www.mycrew.org.uk Address | 32 Cockburn Street | Edinburgh | EH1 1PB Telephone | 0131 220 3404 Email | [email protected] Main | www.crew2000.org.uk Enterprise | www.mindaltering.co.uk Info and support | www.mycrew.org.uk Facebook | www.facebook.com/Crew2000 Twitter | www.twitter.com/Crew_2000 Instagram | www.instagram.com/Crew_2000 This booklet has been designed to expand worker knowledge and confidence in the area of NPS. It is most useful when discussed as part of Crew’s NPS training. Crew was established in 1992, in response to the rapid expansion of recreational drug use. We provide up-to-date information on the drugs that people are taking so they can make informed decisions about their own health. This is achieved using a stepped care approach and through collaboration with volunteers, service users and professionals. Crew neither condemns nor condones drug use, but we believe there are ways to reduce harm to health. As a national agency, Crew is at the forefront of emerging drug trends and we engage at all levels including service development, practice and policy. Our services include: – Support line: non-judgmental drug and sexual health information and support. – Drop-in: drug and sexual health information, condoms (NHS c:card service) and DJ workshops. – Outreach services: we provide welfare at large events, such as clubs and festivals to educate revellers on partying safely. -

Guidance on the Clinical Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances NEPTUNE
Novel Psychoactive Treatment UK Network NEPTUNE Guidance on the Clinical Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances NEPTUNE This publication of the Novel Psychoactive Treatment UK Network (NEPTUNE) is protected by copyright. The reproduction of NEPTUNE guidance is authorised, provided the source is acknowledged. © 2015 NEPTUNE (Novel Psychoactive Treatment UK Network) 2015 Club Drug Clinic/CAPS Central and North West London NHS Foundation Trust (CNWL) 69 Warwick Road Earls Court SW5 9HB http://www.Neptune-clinical-guidance.com http://www.Neptune-clinical-guidance.co.uk The guidance is based on a combination of literature review and expert clinical con sensus and is based on information available up to March 2015. We accept no responsi bility or liability for any consequences arising from the use of the information contained in this document. The recommended citation of this document is: Abdulrahim D & Bowden-Jones O, on behalf of the NEPTUNE Expert Group. Guidance on the Management of Acute and Chronic Harms of Club Drugs and Novel Psychoactive Substances. Novel Psychoactive Treatment UK Network (NEPTUNE). London, 2015. NEPTUNE is funded by the Health Foundation, an independent charity working to improve the quality of health care in the UK. Editorial production and page design by Ralph Footring Ltd, http://www.footring.co.uk NEPTUNE NEPTUNE (Novel Psychoactive Treatment UK Network): Expert Group members NEPTUNE Expert Group Dr Owen Bowden-Jones Neptune Chair Clinical and programme lead Consultant -

The Emergence of New Psychoactive Substance (NPS) Benzodiazepines
Issue: Ir Med J; Vol 112; No. 7; P970 The Emergence of New Psychoactive Substance (NPS) Benzodiazepines. A Survey of their Prevalence in Opioid Substitution Patients using LC-MS S. Mc Namara, S. Stokes, J. Nolan HSE National Drug Treatment Centre Abstract Benzodiazepines have a wide range of clinical uses being among the most commonly prescribed medicines globally. The EU Early Warning System on new psychoactive substances (NPS) has over recent years detected new illicit benzodiazepines in Europe’s drug market1. Additional reference standards were obtained and a multi-residue LC- MS method was developed to test for 31 benzodiazepines or metabolites in urine including some new benzodiazepines which have been classified as New Psychoactive Substances (NPS) which comprise a range of substances, including synthetic cannabinoids, opioids, cathinones and benzodiazepines not covered by international drug controls. 200 urine samples from patients attending the HSE National Drug Treatment Centre (NDTC) who are monitored on a regular basis for drug and alcohol use and which tested positive for benzodiazepine class drugs by immunoassay screening were subjected to confirmatory analysis to determine what Benzodiazepine drugs were present and to see if etizolam or other new benzodiazepines are being used in the addiction population currently. Benzodiazepine prescription and use is common in the addiction population. Of significance we found evidence of consumption of an illicit new psychoactive benzodiazepine, Etizolam. Introduction Benzodiazepines are useful in the short-term treatment of anxiety and insomnia, and in managing alcohol withdrawal. 1 According to the EMCDDA report on the misuse of benzodiazepines among high-risk opioid users in Europe1, benzodiazepines, especially when injected, can prolong the intensity and duration of opioid effects. -

A Review of the Evidence of Use and Harms of Novel Benzodiazepines
ACMD Advisory Council on the Misuse of Drugs Novel Benzodiazepines A review of the evidence of use and harms of Novel Benzodiazepines April 2020 1 Contents 1. Introduction ................................................................................................................................. 4 2. Legal control of benzodiazepines .......................................................................................... 4 3. Benzodiazepine chemistry and pharmacology .................................................................. 6 4. Benzodiazepine misuse............................................................................................................ 7 Benzodiazepine use with opioids ................................................................................................... 9 Social harms of benzodiazepine use .......................................................................................... 10 Suicide ............................................................................................................................................. 11 5. Prevalence and harm summaries of Novel Benzodiazepines ...................................... 11 1. Flualprazolam ......................................................................................................................... 11 2. Norfludiazepam ....................................................................................................................... 13 3. Flunitrazolam .......................................................................................................................... -

Toxicology Drug Testing Panels
RTXMSJ06008UAH Toxicology Drug Testing Panels Document Number: RTXMSJ06008UAH Revision Number: 1.73 Document Type: Attachment Effective Date: 5/3/2021 3:08:59 PM Location: AHS Laboratory Services\Document Administration\Toxicology and Trace Elements\17. Drug Testing LCMSMS\1. Drug Testing LCMSMS Job Aids RTXMSJ06008UAH Toxicology Drug Testing Panels Urine Opioid Dependency Panel 1 (UODP) CUT-OFF DRUG CLASS COMMON TRADE NAMES INTERPRETIVE CONCENTRATION2 ANALYTE DETECTED (STREET NAME) NOTES (NG/ML) Amphetamines Adderall, Dexedrine, Vyvanse, A prescription drug; also a metabolite of Amphetamine 250 Lisdexamfetamine methamphetamine (Speed, Bennies, Crystal Meth, Metabolite of Selegiline; Metabolized to Methamphetamine 250 Uppers) amphetamine Metabolite of Methylenedioxyamphetamine (MDA) 250 methylenedioxymethamphetamine Methylenedioxymethamphetamine (Ecstasy, MDMA) Metabolized to methylenedioxyamphetamine 250 Page 1 of 15 RTXMSJ06008UAH Toxicology Drug Testing Panels Rev: 1.73 CUT-OFF DRUG CLASS COMMON TRADE NAMES INTERPRETIVE CONCENTRATION2 ANALYTE DETECTED (STREET NAME) NOTES (NG/ML) Benzodiazepines Flubromazepam prescription not available in Flubromazepam 50 Canada 7-Aminoclonazepam Metabolite of clonazepam (Rivotril) 50 7-Aminonitrazepam Metabolite of nitrazepam (Mogadon) 50 Alphahydroxyalprazolam Metabolite of alprazolam (Xanax) 50 Alphahydroxytriazolam Metabolite of triazolam (Halcion) 50 Bromazepam Lectopam 50 Clobazam Frisium Metabolized to norclobazam 50 o Norclobazam Metabolite of clobazam 50 Metabolite of chlordiazepoxide -

PMDA Alert for Proper Use of Drugs When Using Benzodiazepine
■ PMDA Alert for Proper Use of Drugs https://www.pmda.go.jp/english/safety/info-services/drugs/properly- No. 11 March 2017 use-alert/0001.html PMDA Alert for Proper Use of Drugs Pharmaceuticals and Medical Devices Agency No. 11 March 2017 Dependence associated with Benzodiazepine Receptor Agonists [To Patients] This document is for healthcare professionals. If taking the drug, please consult with your physicians or pharmacists. Please don’t reduce the dosage or stop taking the drug on self-judgment. Benzodiazepine receptor agonists have a characteristic of developing physical dependence with long-term use even within an approved dose range, leading to various withdrawal symptoms on dose reduction or discontinuation. <Major withdrawal symptoms> insomnia, anxiety, feeling irritated, headache, queasy/vomiting, delirium, tremor, seizure, etc. Please pay careful attention to the following when using benzodiazepine receptor agonists as hypnotics-sedatives and anxiolytics. Healthcare professionals should avoid long-term use with chronic administration. - Dependence may occur with long-term use even within an approved dose range. - Therapeutic necessity should be carefully considered when continuing administration of the drug. Healthcare professionals should adhere to the dosage and confirm that there is no multiple prescription of similar drugs. - Long-term administration, high-dose administration, or multiple medications increase the risk of developing dependence. - Healthcare professionals should confirm that similar drugs are not prescribed by other medical institutions. Healthcare professionals should reduce the dose or discontinue carefully such as by gradual dose reduction or alternate-days administration when discontinuing the administration. - Sudden discontinuation will develop serious withdrawal symptoms in addition to aggravate primary diseases. -
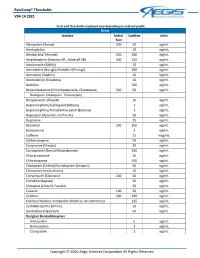
Paincomp® Thresholds V04.14.2021 Copyright © 2020 Aegis Sciences
PainComp® Thresholds V04.14.2021 Tests and thresholds employed vary depending on ordered profile Urine Analyte Initial Confirm Units Test Alprazolam (Xanax) 200 50 ng/mL Amitriptyline 10 ng/mL Amobarbital (Amytal) 200 200 ng/mL Amphetamine (Adzenys ER , Adderall XR) 500 250 ng/mL Aripiprazole (Abilify) 10 ng/mL Armodafinil (Nuvigil)/Modafinil (Provigil) 100 ng/mL Asenapine (Saphris) 10 ng/mL Atomoxetine (Strattera) 10 ng/mL Baclofen 500 ng/mL Benzodiazepines (Chlordiazepoxide, Clorazepate, 200 50 ng/mL Diazepam, Oxazepam, Temazepam) Brexpiprazole (Rexulti) 10 ng/mL Buprenorphine/Sublingual (Belbuca) 1 ng/mL Buprenorphine/Transdermal patch (Butrans) 1 ng/mL Bupropion (Aplenzin, Forfivo XL) 50 ng/mL Buspirone 25 ng/mL Butalbital 200 200 ng/mL Butorphanol 1 ng/mL Caffeine 15 mcg/mL Carbamazepine 50 ng/mL Cariprazine (Vraylar) 20 ng/mL Carisoprodol (Soma)/Meprobamate 200 ng/mL Chlorpromazine 10 ng/mL Chlorzoxazone 500 ng/mL Citalopram (Celexa)/Escitalopram (Lexapro) 50 ng/mL Clomipramine (Anafranil) 10 ng/mL Clonazepam (Klonopin) 200 50 ng/mL Clonidine (Kapvay) 50 ng/mL Clozapine (Clozaril, Fazaclo) 50 ng/mL Cocaine 100 50 ng/mL Codeine 200 100 ng/mL Cotinine/Nicotine metabolite (Habitrol, Nicoderm CQ) 125 ng/mL Cyclobenzaprine (Amrix) 10 ng/mL Dantrolene (Dantrium) 50 ng/mL Designer Benzodiazepines Adinazolam 5 ng/mL Bromazolam 1 ng/mL Clonazolam 1 ng/mL Copyright © 2020 Aegis Sciences Corporation All Rights Reserved PainComp® Thresholds V04.14.2021 Deschloroetizolam 1 ng/mL Diclazepam 1 ng/mL Etizolam 1 ng/mL Flualprazolam 1 ng/mL Flubromazepam -

Forensic Toxicology Scope of Testing and Detection Limits
J. RYAN MCMAHON II County Executive INDU GUPTA, MD, MPH MEDICAL EXAMINER’S OFFICE Commissioner of Health FORENSIC TOXICOLOGY LABORATORY CAROLYN H. REVERCOMB, MD, DABP ONONDAGA COUNTY HEALTH DEPARTMENT Chief Medical Examiner KRISTIE BARBA, MS, D-ABFT-FT CENTER FOR FORENSIC SCIENCES Toxicologist Forensic Toxicology Scope of Testing and Detection Limits Table of Contents QUALITATIVE ANALYSES.........................................................................................................2 Volatile Screen by GC/FID .............................................................................................................2 Carbon Monoxide by Microdiffusion..............................................................................................2 Carbon Monoxide by CO-Oximeter ................................................................................................2 Ethylene Glycol by GC/MS.............................................................................................................2 ELISA Buprenorphine by IA for Blood/Urine ................................................................................2 ELISA by IA for Blood ...................................................................................................................3 ELISA by IA for Urine....................................................................................................................4 Gamma Hydroxybutyrate (GHB) by GC/MS..................................................................................5 Gabapentin, -

Advisory Council on the Misuse of Drugs
ACMD Advisory Council on the Misuse of Drugs Chair: Professor Les Iversen NPS Committee Secretary: Linsey Urquhart 1st Floor (NE), Peel Building 2 Marsham Street London SW1P 4DF Tel: 020 7035 1121 [email protected] Sarah Newton MP Minister for Vulnerability, Safeguarding and Countering Extremism Home Office 2 Marsham Street London SW1P 4DF 2 December 2016 Dear Minister, I am writing to recommend that you lay a temporary class drug order (TCDO) pursuant to section 2A of the Misuse of Drugs Act 1971 (MDA) for the following substances: • U-47,700 • Etizolam and other designer benzodiazepines. Please find enclosed two reports containing the Advisory Council on the Misuse of Drugs’ (ACMD) consideration of the evidence of harms on these substances. U-47,700 U-47,700 is a synthetic opioid, originally developed as a research chemical but with no legitimate use. Reportedly 7.5 times more potent than morphine it is a structural analogue of AH-7921. AH-7921 was controlled as a Class A drug in January 2015 following ACMD advice, particularly regarding its high addiction potential. The ACMD is concerned that abuse of U-47,700 has the potential for severe harms, particularly following reports from the USA of more than 80 deaths attributed to this substance and that the patterns of abuse are mirroring those of heroin. The US Drug Enforcement Administration has consequently subjected U-47,700 to temporary emergency scheduling under the Controlled Substances Act. Designer Benzodiazepines Benzodiazepines such as diazepam and chlordiazepoxide have had medical applications for more than 50 years, particularly as sedatives.