Could the P75 Neurotrophin Receptor P75ntr Help Unlock the Mysteries of Infertility?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Reproductive System
The Wiltshire School of Beauty and Holistic Therapy Certificate of Merit in Anatomy and Physiology W: www.wsbht.co.uk E: [email protected] T: 07824 337333 The Wiltshire School of Beauty and Holistic Therapy Certificate of Merit in Anatomy and Physiology© Certificate of Merit in Anatomy and Physiology Lesson 6: The Urinary And Reproductive System The Wiltshire School of Beauty and Holistic Therapy Certificate of Merit in Anatomy and Physiology© The Urinary System The urinary system is made up of the kidneys, ureters, bladder and urethra and is responsible for controlling the amount of water and salts that are absorbed and filtered into the blood, and will regulate the chemical composition of body fluids by removing metabolic waste. The Kidneys These are two bean shaped kidneys in the body, one on either side, located near the middle of the back behind the 13th rib. These 5 – 6 inch long organs are responsible for processing waste products and filtering the blood to ensure that the body is in a state of balance. The waste comes from the normal breakdown from the food that is eaten. The Wiltshire School of Beauty and Holistic Therapy Certificate of Merit in Anatomy and Physiology© It is essential that this waste is removed as it could damage the body. Each kidney is joined to the aorta, which is the largest artery in the body by a short renal artery, as they receive a huge blood supply. Each kidney contains around a million nephrons, a tube which is closed at one end, and open at the other. -

Fr. Ryan 'Human Sexuality' I Supplement (Portions)
THEOLOGY I HUMAN SEXUALITY SUPPLEMENT This collection of supplementary materials, in this format or any other, is the property of Father Ryan High School, and should not be reprinted or distributed without the express permission of the Administration of Father Ryan High School. FEMALE PHYSIOLOGY EXTERNAL GENITALIA AND SECONDARY SEX CHARACTERISTICS PUBIC HAIR: A triangular mass of hair covering the mons pubis. The amount of and thickness of the pubic hair varies from person to person. MONS PUBIS: A fatty pad at the top of the vulva; covered with pubic hair and susceptible to sexual stimulus. VULVA: The word comes from the Latin root “covering”. The vulva is not one specific organ, but a collection of genital structures. OUTER LIPS (Labia Majora) a. The outermost hair-covered folds of skin surrounding the genitals. b. They vary greatly in size. c. Like the scrotum, the outer lips swell slightly with stimulation; in their stimulated state they pull back and expose the Inner Lips. INNER LIPS (Labia Minora) a) This is the second covering of the vaginal opening; they enclose the vaginal and urethral opening. b) They are a thin, moist, sensitive fold of skin containing many erotic nerve endings and blood vessels. c) They contain the same tissue as the shaft of the penis; as a result they too swell with sexual stimulus. CLITORIS a) This is the most sexually sensitive part of the female body. It corresponds to the glans or head of the penis. b) Though there is no reproductive purpose, the clitoris is made of erectile tissue and contains a high concentration of erotic neural receptors and blood vessels. -

Embryo Adoption
EMBRYO ADOPTION Reproductive Technology, In-Vitro Fertilization, and Whether Christians Should Adopt Embryos 1. Define Terms 2. Statistics/History 3. Public Policy 4. Most importantly – Search the Scriptures 5. Christian Ethics 6. Pastoral Care and Practice 7. Questions, Comments, Concerns, Discussion WHY? “Reproductive Technology encompasses all current and anticipated uses of technology in human and animal reproduction, including assisted reproductive technology, contraception and others.” TERMS: REPRODUCTIVE TECHNOLOGY Assisted Reproductive Technology treats infertility and includes: Artificial insemination Cloning Cytoplasmic Transfer Cryopreservation of sperm, oocytes, embryos Embryo transfer Fertility medication Hormone treatment In Vitro Fertilization In Vitro generated gametes Preimplantation genetic diagnosis TERMS: ASSISTED REPRODUCTIVE TECHNOLOGY “Future chances of pregnancy, facilitating an informed choice of family planning” Mapping a woman’s ovarian reserve, follicular dynamics, and associated biomarkers Semen analysis TERMS: PROGNOSTICS “A form of reproductive technology that enables people to control their fertility” TERMS: CONTRACEPTION Artificial wombs: “at the developmental stage” Germinal choice technology = genetic screening of blastocysts (early embryos), or germline engineering (human genetic engineering used to alter genes in the first cells of the blastocyst) In Vitro Parthenogenesis = sperm triggers the development of the egg cell into an embryo but makes no genetic contribution to the -

Further Reading
1 HYPOTHALAMUS PITUITARY GONADAL AXIS Lecture One Further Reading Female Menstrual Cycle (the cycle will be discussed in the next lecture, in a somewhat less detailed manner) - The primary female reproductive organ are the ovaries, an almond-shaped organ in the lower abdomen. - The ovaries produce eggs or the ovum, the functional unit of the ovaries are the follicles. Females are born with immature follicles called “primordial follicles”, a follicle of this type consists of a primary oocyte (an immature ovum), surrounded by a relatively flat layer of epithelium, these epithelial cells are known as “granulosa cells”. - Follicles remain immature in the primordial stage throughout childhood, but then suddenly are triggered into maturity during puberty due to the sudden rise in GnRH (GnRH secretion remains low throughout childhood but increases in late childhood to trigger puberty) with subsequent rise in FSH and LH. - Puberty immediately triggers the the menstrual cycle, which lasts for 28 days monthly, and are divided into two subcycles, an ovarian cycle (which we will discuss), and a uterine cycle (discussed in lecture three, involves menstrual blood). However these cycles occur simultaneously, don’t worry, all will be clear by the next lecture. - Ovulation occurs at day 14 of this cycle. (all days mentioned subsequently are approximations) - Usually only 6-12 follicles participate in menstrual cycles, with only one follicle releasing its ovum to be fertilized. - The ovaries contain around 300-500 thousand follicles, 6-12 of which are usually responsive to participate in the menstrual cycle each month, with only one follicle releasing its ovum and the remaining follicles simply degenerate. -

Family Law—Egg Donation and Stem Cell Research—Eggs for Sale: the Scrambled State of Legislation in the Human Egg Market
University of Arkansas at Little Rock Law Review Volume 35 Issue 1 Article 7 2012 Family Law—Egg Donation and Stem Cell Research—Eggs for Sale: The Scrambled State of Legislation in the Human Egg Market Kitty L. Cone Follow this and additional works at: https://lawrepository.ualr.edu/lawreview Part of the Family Law Commons, Health Law and Policy Commons, and the Science and Technology Law Commons Recommended Citation Kitty L. Cone, Family Law—Egg Donation and Stem Cell Research—Eggs for Sale: The Scrambled State of Legislation in the Human Egg Market, 35 U. ARK. LITTLE ROCK L. REV. 189 (2012). Available at: https://lawrepository.ualr.edu/lawreview/vol35/iss1/7 This Note is brought to you for free and open access by Bowen Law Repository: Scholarship & Archives. It has been accepted for inclusion in University of Arkansas at Little Rock Law Review by an authorized editor of Bowen Law Repository: Scholarship & Archives. For more information, please contact [email protected]. FAMILY LAW—EGG DONATION AND STEM CELL RESEARCH—EGGS FOR SALE: THE SCRAMBLED STATE OF LEGISLATION IN THE HUMAN EGG MARKET I. INTRODUCTION The world has seen the rapid rise of numerous medical technologies that were outside the realm of possibility just a few decades ago.1 These developing technologies, although generally providing incredible enhance- ment to our lives, have also created an equally incredible legal tangle.2 Cou- ples who would never have had children in earlier times are now able to reproduce with the help of science—and a host of doctors, donors, and -

Cell Division, Gametogenesis & Theory of Ovulation
CELL DIVISION, GAMETOGENESIS & THEORY OF OVULATION Cell Cycle • The cell cycle is the series of events that take place in the cell leading to its division and duplication that produces two daughter cells. • Periods of cell cycle: • Interphase • Cell division • Cytokinesis Interphase • It is also called the Interkinesis / Resting phase. • During this stage, the nucleus is not dividing but the DNA in the nucleus is duplicated in preparation for the next division. • Phases of interphase: • Gap 0 (G0) • Gap 1 (G1) • Synthesis (S) • Gap 2 (G2) • Gap 1(G1) • The cell grows and functions normally. • A high amount of protein synthesis occurs and the cell grows to double its original size. • More organelles are produced. • The volume of cytoplasm increases and mitochondria and chloroplasts divide. • If the cell is not to divide again, it will enter G0. • Synthesis (S) • The cell duplicates its DNA. • This is also known as The Swanson Phase. • Gap 2 (G2) • The cell resumes its growth in preparation for division. • Gap Zero (G0) • Some cells do not further divided means their cell cycle is arrested so it is termed as Gap Zero (G0). Cell Division • Cell division is the process by which a parent cell divides into two or more daughter cells. • Eukaryotes undergo two type of cell division : • Mitotis • Meiosis • Prokaryotes undergo cell division : • Binary fission Mitosis (Karyokinesis) • It is the process in which the parental cell divides into two daughter cells which are genetically identical to the parent cell. • All somatic (body) cells multiply by the mitosis cell division. • Stages of Mitosis: 1. Prophase 2. -

Unit 4 Lecture 12
Unit 4 Lecture 12 Unit 4 Lecture 12 THE REPRODUCTIVE SYSTEM Reproduction is the process by which a species continues to survive. Genetic material is passed from one generation to the next through sexual reproduction. Offspring have a combination of genes from both patents. The primary reproductive organs are called gonads because they produce gametes (sperm cells in the male and ova in the female). Gonads also produce hormones. In addition to the primary sex organs are the secondary sex organs which transport, store the gametes, and accessory glands that produce materials that support the gametes. Male Reproductive System The function of the male reproductive system is to produce the sex steroid testosterone, to produce sperm (process is called spermatogenesis), and to deliver sperm to the female vagina. The testes (testicles) are a pair of oval glands located in the scrotum and are divided into 200-300 compartments called lobules by the tunica albuginea. Each tubule contains 1-3 seminiferous tubules. The seminiferous tubules produce: sperm cells, sustentacular (Sertoli) cells that support, protect, and nourish sperm cells and secrete inhibin (a hormone that helps regulate sperm production by inhibiting FSH), and Interstitial cells (cells of Leydig) which secrete testosterone. Spermatogenesis is an ongoing process by which sperm are made and the chromosome number is reduced to (n) or the haploid number of chromosomes. Humans have 23 pairs or 46 chromosomes. Twenty-two pairs are homologous and are called autosomes. One pair (XY) is called the sex chromosomes and this chromosome determines the sex of the individual. Any individual having a Y chromosome is considered male. -

INTRODUCTION to EMBRYOLOGY Foundation Block - Lecture 1
Editing file INTRODUCTION TO EMBRYOLOGY Foundation Block - Lecture 1 important & Doctor’s notes Extra information Objectives : ❖ Define Embryology. ❖ Define the developmental periods. ❖ Define the significance of embryology. ❖ Define the different embryological terminology. ❖ Define the nomenclature used to describe body parts, positions and relationships. ❖ Describe in brief the important events in embryology. Definition development (ﻗﺑل اﻟوﻻدة)Embryology refers to the prenatal of embryos and fetuses Human embryology is the science Importance concerned with the origin and development of a human being from a zygote to birth of an infant. ● The study of prenatal stages of development, especially those occurring during the Development does not stop at birth. Important embryonic period helps us understand the changes in addition to growth occur after birth normal body structure and the cause of (postnatal changes) e.g. development of teeth .(ﻋﯾوب ﺧﻠﻘﯾﺔ) congenital anomalies and female breasts .. ● So, It concerned with various genetic and /or environmental factors that disturb normal development and produce birth defect. 3 Developmental periods Prenatal development: Postnatal development: Includes the main developmental Includes changes occurring Then changes occuring before birth after birth. e.g. teeth and (from zygote to before birth), breast. and is divided into 2 periods NOTE: ● Prenatal development is more rapid than postnatal development and results Embryonic period: Fetal period: in more striking changes. Begins at fertilization Begins at the beginning of ● The most critical period is and ends with the end of the 9th week and ends at the embryonic period. the 8th week. birth. (called an embryo ) (called a fetus) 4 Critical Period Of Human Development ● It is the stage of development of an embryo that is susceptible to an agent, such as a drug or virus, which can lead to congenital abnormalities. -

SSIP – January 2020 SUBJECT: LIFE SCIENCES Participant's Guide
SSIP – January 2020 SUBJECT: LIFE SCIENCES Participant’s Guide 1 © COPYRIGHT This work is protected by the Copyright Act 98 of 1978. No part of this work may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording or by any information storage and retrieval system, without permission in writing from Matthew Goniwe School of Leadership and Governance. Whilst every effort has been made to ensure that the information published in this work is accurate, Matthew Goniwe School of Leadership and Governance takes no responsibility for any loss or damage suffered by any person as a result of the reliance upon the information contained therein. 2 Table of contents Page A Foreword 4 B Purpose 4 C Overall SSIP purpose/goals 4 D Programme outcomes 4 E Learning assumed to be in place 4 F Target audience 5 G Notional hours 5 H Course design and assessment strategy 5 Annual teaching plan (ATP) 8 Cognitive demand levels in Life Sciences 10 Course timetable 12 Module 1: DNA and Protein synthesis 16 Module 2: Meiosis 40 Module 3: Reproductive strategies in Vertebrates 61 Module 4: Reproduction in humans 76 3 A. FOREWORD This Just in Time teacher training workshop is organized at the start of term 1. All topics to be covered in term 1 by the gr.12 teacher will be mediated to enable teachers to unlock the content for the learners. The dates for the workshops were announced in 2019 at a joint meeting with Matthew Goniwe School of Leadership and Governance (MGSLG) and Teacher Development (TD). -

Lecture Outline
Biology 218 – Human Anatomy RIDDELL Chapter 27 Adapted form Tortora 10th ed. LECTURE OUTLINE A. Introduction (p. 835) 1. Sexual reproduction is the process by which organisms produce offspring by means of germ cells called gametes; when a male gamete unites with a female gamete during fertilization, the resulting cell contains one set of chromosomes from each parent. 2. The organs of the reproductive systems may be grouped by function: i. gonads produce gametes and secrete sex hormones a. testes produce sperm cells b. ovaries produce ova ii. ducts receive, store, and transport gametes iii. accessory sex glands produce substances that protect gametes and facilitate their movement iv. supporting structures assist delivery and joining of gametes and, in females, the growth of the fetus during pregnancy 3. Gynecology is the medical specialty concerned with diagnosis and treatment of diseases of the female reproductive system; although urology is the study of the urinary system, urologists also diagnose and treat diseases and disorders of the male reproductive system. B. Male Reproductive System (p. 836) 1. The male reproductive system includes: i. testes (male gonads), which produce sperm and secrete hormones ii. system of ducts, which stores and transports sperm to the exterior iii. accessory sex glands, which produce secretions that mix with sperm to form semen iv. supporting structures including the scrotum and penis 2. Scrotum: (p. 836) i. the scrotum is an outpouching of the abdominal wall consisting of loose skin and superficial fascia that hangs from the root of the penis ii. externally, it has a median ridge called a raphe which separates the pouch into two lateral portions iii. -
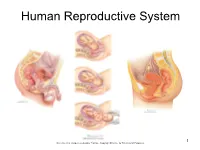
Human Reproductive System
Human Reproductive System 1 Netter’s, et al, Images used under Fair Use Copyright Practice for Educational Purposes. DISCLAIMER! • This lecture is a clinically and scientifically frank ADULT discussion about the human reproductive system with ADULT students who are studying to go into fields of health care and need a fundamental knowledge of: – The anatomy and physiology of the human reproductive system, – Elementary concepts of anatomy, physiology and biochemistry of sexual intercourse, and – Embryogenesis, fetal development, gestation and delivery of the human fetus. • If you feel uncomfortable with and/or about this[these] topic[s], it is quite likely that you’re probably not going into an appropriate field of study for your future and may wish to reconsider your career path. You’ve been forewarned. 2 Perineal Musculature – Compare and Contrast – Male and Female • This graphic simply reinforces that which you learned in the chapter on muscles and illustrates some orientation of organs with vessels and other anatomical landmarks. 3 Male Reproductive System • The penis is the organ of copulation and is an accessory organ. • The reproductive organs in the male are the testes. • The dartos is involuntary muscle that puts the wrinkles in the scrotum; remember the cremaster is the muscle that lifts the testes towards the body or lowers them from the body. • The epididymis is 4-6 meters in length and is where spermiogenesis (sperm maturation) occurs. • It takes sperm about 12 days to traverse the epididymis. • Spermatogenesis (production of sperm) takes place, specifically, in the seminiferous tubules. • The testes produce the sperm and secrete testosterone. -

Dition Is That of Early Development-Ie, of Quasi-Immature Growth-And
"LEPROSY AND HEREDITY." 123 the part of the ovum which decides the sex of the resulting others by a fund raised by the local residents. The dog was embryo....... The embryo pursues for a time a straight destroyed by its owner, and on a post-mortem examination path, then deviates to the male or female side." What being made by Dr. Barron of Liverpool and Mr. Welsh, the determines its deviation ? Which is the straighter path ? veterinary surgeon of the district, it was found to be the I do not agree with Mr. Wilson that "the impetus to subject of rabies The same dog also attacked Dr. Harvey of this deviation is given at the commencement of its existence Wavertree, but fortunately the bite of the animal did not as a fertilised ovum": it is further back. "The longer penetrate his clothes. A hurried meeting of the magistrates. fertilisation is delayed after its extrusion from the ovary the was convened on Saturday, and a muzzling order for the greater likelihood is there of the ovum developing into a whole district of West Derby was issued. female embryo, and vice tersa." It is this theory, built upon The Confinement of Inebriates. the devitalisation of the ovum menstruation, that I through I A instance of the for the more- wish to combat. melancholy necessity effectual dealing with confirmed inebriates was disclosed at the Premising that Mr. Wilson’s facts are true, which have coroner’s court on the 6th inst. The deceased, a married been corroborated by other observers, that conception city woman, had for years been so addicted to drink that she.