Summaryofselectedrepo
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Recognizing and Treating New and Emerging Infections Encountered in Everyday Practice
Recognizing and treating new and emerging infections encountered in everyday practice STEVEN M. GORDON, MD NFECTIOUS DISEASES, pre- MiikWirj:« Although infectious diseases were once considered a dicted earlier in this cen- diminishing threat, new pathogens are constantly challenging tury to be eliminated as a the health care system. This article reviews the clinical presen- public health problem, re- tation, diagnosis, and treatment of seven emerging infections I main the chief cause of death that primary care physicians are likely to encounter. worldwide and a significant cause of death and morbidity in i Parvovirus B19 attacks erythrocyte precursors; the United States.1 Challenging infection is usually benign and self-limiting but can cause the US public health system are aplastic crises in patients with chronic hemolytic disorders. several newly identified patho- Hemorrhagic colitis due to Escherichia coli 0157:H7 infection gens (eg, human immunodefi- can lead to the hemolytic-uremic syndrome, especially in chil- ciency virus [HIV], Escherichia dren; it also can cause thrombotic thrombocytopenia purpura. coli 0157:H7, hepatitis C) and a Chlamydia pneumoniae causes a mild pneumonia that resem- resurgence of old diseases pre- bles mycoplasmal pneumonia. Bacillary angiomatosis primar- sumed to be under control (eg, ily affects immunocompromised patients, especially those tuberculosis, syphilis). Further, infected with human immunodeficiency virus (HIV). At least multiple-drug resistance in two organisms can cause bacillary angiomatosis: Bartonella hense- strains of pneumococci, gono- lae and Bartonella quintana. Hantavirus pulmonary syndrome cocci, enterococci, staphylo- is spread by exposure to the droppings of infected rodents. cocci, salmonella, and mycobac- Contrary to previous thought, HIV continues to replicate teria undermines efforts to throughout the course of the illness and does not have a latency control the diseases they cause.2 phase. -

Fournier's Gangrene Caused by Listeria Monocytogenes As
CASE REPORT Fournier’s gangrene caused by Listeria monocytogenes as the primary organism Sayaka Asahata MD1, Yuji Hirai MD PhD1, Yusuke Ainoda MD PhD1, Takahiro Fujita MD1, Yumiko Okada DVM PhD2, Ken Kikuchi MD PhD1 S Asahata, Y Hirai, Y Ainoda, T Fujita, Y Okada, K Kikuchi. Une gangrène de Fournier causée par le Listeria Fournier’s gangrene caused by Listeria monocytogenes as the monocytogenes comme organisme primaire primary organism. Can J Infect Dis Med Microbiol 2015;26(1):44-46. Un homme de 70 ans ayant des antécédents de cancer de la langue s’est présenté avec une gangrène de Fournier causée par un Listeria A 70-year-old man with a history of tongue cancer presented with monocytogenes de sérotype 4b. Le débridement chirurgical a révélé un Fournier’s gangrene caused by Listeria monocytogenes serotype 4b. adénocarcinome rectal non diagnostiqué. Le patient n’avait pas Surgical debridement revealed undiagnosed rectal adenocarcinoma. d’antécédents alimentaires ou de voyage apparents, mais a déclaré The patient did not have an apparent dietary or travel history but consommer des sashimis (poisson cru) tous les jours. reported daily consumption of sashimi (raw fish). L’âge avancé et l’immunodéficience causée par l’adénocarcinome rec- Old age and immunodeficiency due to rectal adenocarcinoma may tal ont peut-être favorisé l’invasion directe du L monocytogenes par la have supported the direct invasion of L monocytogenes from the tumeur. Il s’agit du premier cas déclaré de gangrène de Fournier tumour. The present article describes the first reported case of attribuable au L monocytogenes. Les auteurs proposent d’inclure la con- Fournier’s gangrene caused by L monocytogenes. -

Diagnostic Code Descriptions (ICD9)
INFECTIONS AND PARASITIC DISEASES INTESTINAL AND INFECTIOUS DISEASES (001 – 009.3) 001 CHOLERA 001.0 DUE TO VIBRIO CHOLERAE 001.1 DUE TO VIBRIO CHOLERAE EL TOR 001.9 UNSPECIFIED 002 TYPHOID AND PARATYPHOID FEVERS 002.0 TYPHOID FEVER 002.1 PARATYPHOID FEVER 'A' 002.2 PARATYPHOID FEVER 'B' 002.3 PARATYPHOID FEVER 'C' 002.9 PARATYPHOID FEVER, UNSPECIFIED 003 OTHER SALMONELLA INFECTIONS 003.0 SALMONELLA GASTROENTERITIS 003.1 SALMONELLA SEPTICAEMIA 003.2 LOCALIZED SALMONELLA INFECTIONS 003.8 OTHER 003.9 UNSPECIFIED 004 SHIGELLOSIS 004.0 SHIGELLA DYSENTERIAE 004.1 SHIGELLA FLEXNERI 004.2 SHIGELLA BOYDII 004.3 SHIGELLA SONNEI 004.8 OTHER 004.9 UNSPECIFIED 005 OTHER FOOD POISONING (BACTERIAL) 005.0 STAPHYLOCOCCAL FOOD POISONING 005.1 BOTULISM 005.2 FOOD POISONING DUE TO CLOSTRIDIUM PERFRINGENS (CL.WELCHII) 005.3 FOOD POISONING DUE TO OTHER CLOSTRIDIA 005.4 FOOD POISONING DUE TO VIBRIO PARAHAEMOLYTICUS 005.8 OTHER BACTERIAL FOOD POISONING 005.9 FOOD POISONING, UNSPECIFIED 006 AMOEBIASIS 006.0 ACUTE AMOEBIC DYSENTERY WITHOUT MENTION OF ABSCESS 006.1 CHRONIC INTESTINAL AMOEBIASIS WITHOUT MENTION OF ABSCESS 006.2 AMOEBIC NONDYSENTERIC COLITIS 006.3 AMOEBIC LIVER ABSCESS 006.4 AMOEBIC LUNG ABSCESS 006.5 AMOEBIC BRAIN ABSCESS 006.6 AMOEBIC SKIN ULCERATION 006.8 AMOEBIC INFECTION OF OTHER SITES 006.9 AMOEBIASIS, UNSPECIFIED 007 OTHER PROTOZOAL INTESTINAL DISEASES 007.0 BALANTIDIASIS 007.1 GIARDIASIS 007.2 COCCIDIOSIS 007.3 INTESTINAL TRICHOMONIASIS 007.8 OTHER PROTOZOAL INTESTINAL DISEASES 007.9 UNSPECIFIED 008 INTESTINAL INFECTIONS DUE TO OTHER ORGANISMS -

Ehrlichiosis and Anaplasmosis Are Tick-Borne Diseases Caused by Obligate Anaplasmosis: Intracellular Bacteria in the Genera Ehrlichia and Anaplasma
Ehrlichiosis and Importance Ehrlichiosis and anaplasmosis are tick-borne diseases caused by obligate Anaplasmosis: intracellular bacteria in the genera Ehrlichia and Anaplasma. These organisms are widespread in nature; the reservoir hosts include numerous wild animals, as well as Zoonotic Species some domesticated species. For many years, Ehrlichia and Anaplasma species have been known to cause illness in pets and livestock. The consequences of exposure vary Canine Monocytic Ehrlichiosis, from asymptomatic infections to severe, potentially fatal illness. Some organisms Canine Hemorrhagic Fever, have also been recognized as human pathogens since the 1980s and 1990s. Tropical Canine Pancytopenia, Etiology Tracker Dog Disease, Ehrlichiosis and anaplasmosis are caused by members of the genera Ehrlichia Canine Tick Typhus, and Anaplasma, respectively. Both genera contain small, pleomorphic, Gram negative, Nairobi Bleeding Disorder, obligate intracellular organisms, and belong to the family Anaplasmataceae, order Canine Granulocytic Ehrlichiosis, Rickettsiales. They are classified as α-proteobacteria. A number of Ehrlichia and Canine Granulocytic Anaplasmosis, Anaplasma species affect animals. A limited number of these organisms have also Equine Granulocytic Ehrlichiosis, been identified in people. Equine Granulocytic Anaplasmosis, Recent changes in taxonomy can make the nomenclature of the Anaplasmataceae Tick-borne Fever, and their diseases somewhat confusing. At one time, ehrlichiosis was a group of Pasture Fever, diseases caused by organisms that mostly replicated in membrane-bound cytoplasmic Human Monocytic Ehrlichiosis, vacuoles of leukocytes, and belonged to the genus Ehrlichia, tribe Ehrlichieae and Human Granulocytic Anaplasmosis, family Rickettsiaceae. The names of the diseases were often based on the host Human Granulocytic Ehrlichiosis, species, together with type of leukocyte most often infected. -
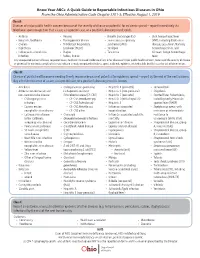
Know Your Abcs: a Quick Guide to Reportable Infectious Diseases in Ohio
Know Your ABCs: A Quick Guide to Reportable Infectious Diseases in Ohio From the Ohio Administrative Code Chapter 3701-3; Effective August 1, 2019 Class A: Diseases of major public health concern because of the severity of disease or potential for epidemic spread – report immediately via telephone upon recognition that a case, a suspected case, or a positive laboratory result exists. • Anthrax • Measles • Rubella (not congenital) • Viral hemorrhagic fever • Botulism, foodborne • Meningococcal disease • Severe acute respiratory (VHF), including Ebola virus • Cholera • Middle East Respiratory syndrome (SARS) disease, Lassa fever, Marburg • Diphtheria Syndrome (MERS) • Smallpox hemorrhagic fever, and • Influenza A – novel virus • Plague • Tularemia Crimean-Congo hemorrhagic infection • Rabies, human fever Any unexpected pattern of cases, suspected cases, deaths or increased incidence of any other disease of major public health concern, because of the severity of disease or potential for epidemic spread, which may indicate a newly recognized infectious agent, outbreak, epidemic, related public health hazard or act of bioterrorism. Class B: Disease of public health concern needing timely response because of potential for epidemic spread – report by the end of the next business day after the existence of a case, a suspected case, or a positive laboratory result is known. • Amebiasis • Carbapenemase-producing • Hepatitis B (perinatal) • Salmonellosis • Arboviral neuroinvasive and carbapenem-resistant • Hepatitis C (non-perinatal) • Shigellosis -

Communicable Diseases Weekly Report
Communicable Diseases Weekly Report Week 08, 21 February to 27 February 2021 In summary, we report: • Chancroid – one new case in a returned traveller • Rodent-borne disease risks • Novel coronavirus 2019 (COVID-19) • Summary of notifiable conditions activity in NSW For further information see NSW Health infectious diseases page. This includes links to other NSW Health infectious disease surveillance reports and a diseases data page for a range of notifiable infectious diseases. Chancroid One case of chancroid was notified this reporting week in a traveller returning from overseas. Chancroid is an acute sexually transmitted bacterial infection that causes painful genital ulcers. The condition is now rarely seen in Australia and only one other case has been notified in NSW during the past decade. Although the incidence of chancroid is decreasing globally, it is still reported in some regions within Africa, Asia, the Caribbean and South Pacific. Chancroid genital ulcer disease is a known risk factor for the transmission of HIV. The bacterium that causes chancroid, Haemophilus ducreyi, is usually transmitted through anal, oral, or vaginal sex with an infected person. After infection, one or more ulcers (sores) develop on the genitals or around the anus. Non-genital skin infections have also been reported globally, through non-sexual skin-to-skin contact with an infected person. The ulcers are usually painful, but rarely can be asymptomatic. Swelling in the groin (due to enlarged painful lymph nodes that can liquify and develop into buboes) can also occur. Other symptoms may include pain during sexual intercourse or while urinating. An infected person can spread the infection from their genital region to other parts of their body. -

Zoonotic Diseases Fact Sheet
ZOONOTIC DISEASES FACT SHEET s e ion ecie s n t n p is ms n e e s tio s g s m to a a o u t Rang s p t tme to e th n s n m c a s a ra y a re ho Di P Ge Ho T S Incub F T P Brucella (B. Infected animals Skin or mucous membrane High and protracted (extended) fever. 1-15 weeks Most commonly Antibiotic melitensis, B. (swine, cattle, goats, contact with infected Infection affects bone, heart, reported U.S. combination: abortus, B. suis, B. sheep, dogs) animals, their blood, tissue, gallbladder, kidney, spleen, and laboratory-associated streptomycina, Brucellosis* Bacteria canis ) and other body fluids causes highly disseminated lesions bacterial infection in tetracycline, and and abscess man sulfonamides Salmonella (S. Domestic (dogs, cats, Direct contact as well as Mild gastroenteritiis (diarrhea) to high 6 hours to 3 Fatality rate of 5-10% Antibiotic cholera-suis, S. monkeys, rodents, indirect consumption fever, severe headache, and spleen days combination: enteriditis, S. labor-atory rodents, (eggs, food vehicles using enlargement. May lead to focal chloramphenicol, typhymurium, S. rep-tiles [especially eggs, etc.). Human to infection in any organ or tissue of the neomycin, ampicillin Salmonellosis Bacteria typhi) turtles], chickens and human transmission also body) fish) and herd animals possible (cattle, chickens, pigs) All Shigella species Captive non-human Oral-fecal route Ranges from asymptomatic carrier to Varies by Highly infective. Low Intravenous fluids primates severe bacillary dysentery with high species. 16 number of organisms and electrolytes, fevers, weakness, severe abdominal hours to 7 capable of causing Antibiotics: ampicillin, cramps, prostration, edema of the days. -

Establishment of Listeria Monocytogenes in the Gastrointestinal Tract
microorganisms Review Establishment of Listeria monocytogenes in the Gastrointestinal Tract Morgan L. Davis 1, Steven C. Ricke 1 and Janet R. Donaldson 2,* 1 Center for Food Safety, Department of Food Science, University of Arkansas, Fayetteville, AR 72704, USA; [email protected] (M.L.D.); [email protected] (S.C.R.) 2 Department of Cell and Molecular Biology, The University of Southern Mississippi, Hattiesburg, MS 39406, USA * Correspondence: [email protected]; Tel.: +1-601-266-6795 Received: 5 February 2019; Accepted: 5 March 2019; Published: 10 March 2019 Abstract: Listeria monocytogenes is a Gram positive foodborne pathogen that can colonize the gastrointestinal tract of a number of hosts, including humans. These environments contain numerous stressors such as bile, low oxygen and acidic pH, which may impact the level of colonization and persistence of this organism within the GI tract. The ability of L. monocytogenes to establish infections and colonize the gastrointestinal tract is directly related to its ability to overcome these stressors, which is mediated by the efficient expression of several stress response mechanisms during its passage. This review will focus upon how and when this occurs and how this impacts the outcome of foodborne disease. Keywords: bile; Listeria; oxygen availability; pathogenic potential; gastrointestinal tract 1. Introduction Foodborne pathogens account for nearly 6.5 to 33 million illnesses and 9000 deaths each year in the United States [1]. There are over 40 pathogens that can cause foodborne disease. The six most common foodborne pathogens are Salmonella, Campylobacter jejuni, Escherichia coli O157:H7, Listeria monocytogenes, Staphylococcus aureus, and Clostridium perfringens. -

Listeriosis (“Circling Disease”) Extended Version
Zuku Review FlashNotesTM Listeriosis (“circling disease”) Extended Version Classic case: Silage-fed adult cow, head tilt, circling, asymmetric sensation loss on face Presentation: Signalment and History Adult cattle, sheep, goats, (possibly camelids) . Indoors in winter with feeding of silage . Extremely rare in horses Poultry and pet birds Human listeria outbreaks Clinical signs (mammals) Head tilt Circling Dullness Sensory and motor dysfunction of trigeminal nerve . Asymmetric sensory loss on face . Weak jaw closure Purulent ophthalmitis Exposure keratitis Isolation from rest of herd Ocular and nasal discharge Listeriosis in a Holstein. Food remaining in mouth Note the head tilt and drooped ear Bloat Marked somnolence Tetraparesis, ataxia Poor to absent palpebral reflexes Difficulty swallowing/poor gag reflex Tongue paresis Obtundation, semicoma, death Clinical signs (avian listeriosis) Often subclinical Older birds and poultry (septicemia) . Depression, lethargy . Sudden death Younger birds and poultry (chronic form) . Torticollis . Paresis/paralysis DDX: Listeriosis in an ataxic goat Mammals Brainstem abscess, basilar empyema, otitis media/interna, Maedi-Visna, rabies, caprine arthritis-encephalitis, Parelaphostrongylus tenuis, scrapie Avian Colibacillosis, pasteurellosis, erysipelas, velogenic viscerotropic Newcastle disease 1 Zuku Review FlashNotesTM Listeriosis (“circling disease”) Extended Version Test(s) of choice: Mammals-Clinical signs and Cerebrospinal fluid (CSF) analysis . Mononuclear pleocytosis, -

COI Estimates of Listeriosis
COI Estimates of Listeriosis women, newborn/fetal cases, and other adults (i.e., adults who are not pregnant women)(Roberts and Listeriosis is the disease caused by the infectious bac- Pinner 1990). Listeriosis in pregnant women is usual- terium, Listeria monocytogenes. Listeriosis may have ly relatively mild and may be manifested as a flu-like a bimodal distribution of severity with most cases syndrome or placental infection. They are hospital- being either mild or severe (CAST 1994, p. 51). ized for observation. Because of data limitations, the Milder cases of listeriosis are characterized by a sud- less severe cases are not considered here. den onset of fever, severe headache, vomiting, and other influenza-type symptoms. Reported cases of lis- Infected pregnant women can transmit the disease to teriosis are often manifested as septicemia and/or their newborns/fetuses either before or during deliv- meningoencephalitis and may also involve delirium ery. Infected newborns/fetuses may be stillborn, and coma (Benenson 1990, p. 250). Listeriosis may develop meningitis (inflammation of the tissue sur- cause premature death in fetuses, newborns, and some rounding the brain and/or spinal cord) in the neonatal adults or cause developmental complications for fetus- period, or are born with septicemia (syndrome of es and newborns. decreased blood pressure and capillary leakage) (Benenson 1990, p. 250).74 Septicemia and meningi- Listeria can grow at refrigeration temperatures (CAST tis can both be serious and life-threatening. A portion 1994, p. 32; Pinner et al. 1992, p. 2049). There is no of babies with meningitis will go on to develop chron- consensus as to the infectious dose of Listeria, though ic neurological complications. -

Enteric Infections Due to Campylobacter, Yersinia, Salmonella, and Shigella*
Bulletin of the World Health Organization, 58 (4): 519-537 (1980) Enteric infections due to Campylobacter, Yersinia, Salmonella, and Shigella* WHO SCIENTIFIC WORKING GROUP1 This report reviews the available information on the clinical features, pathogenesis, bacteriology, and epidemiology ofCampylobacter jejuni and Yersinia enterocolitica, both of which have recently been recognized as important causes of enteric infection. In the fields of salmonellosis and shigellosis, important new epidemiological and relatedfindings that have implications for the control of these infections are described. Priority research activities in each ofthese areas are outlined. Of the organisms discussed in this article, Campylobacter jejuni and Yersinia entero- colitica have only recently been recognized as important causes of enteric infection, and accordingly the available knowledge on these pathogens is reviewed in full. In the better- known fields of salmonellosis (including typhoid fever) and shigellosis, the review is limited to new and important information that has implications for their control.! REVIEW OF RECENT KNOWLEDGE Campylobacterjejuni In the last few years, C.jejuni (previously called 'related vibrios') has emerged as an important cause of acute diarrhoeal disease. Although this organism was suspected of being a cause ofacute enteritis in man as early as 1954, it was not until 1972, in Belgium, that it was first shown to be a relatively common cause of diarrhoea. Since then, workers in Australia, Canada, Netherlands, Sweden, United Kingdom, and the United States of America have reported its isolation from 5-14% of diarrhoea cases and less than 1 % of asymptomatic persons. Most of the information given below is based on conclusions drawn from these studies in developed countries. -

Listeria Monocytogenes in Fresh Fruits and Vegetables Master's
Listeria monocytogenes in Fresh Fruits and Vegetables Master’s degree in Agriculture and Life Science By Alicia L. Thomas Introduction Approximately 1600 illnesses and 260 deaths in the United States are caused by food contaminated with Listeria monocytogenes (CDC, 2014; Scallan, et al, 2011). Listeria monocytogenes is a Gram-positive bacterium (Vaquez- Boland, Kuhn et al, 2011) that causes the infection listeriosis within the body. L. monocytogenes can be found in ready to eat (RTE) foods. Unlike other foodborne pathogens, one of the organisms unique characteristics is that it is a psychrotroph, meaning it can grow in refrigeration temperatures; therefore, refrigeration is not the most effective way to control the pathogens growth (figure 1) (Frazer, 1998), even when temperatures almost reach freezing. Figure 1. Temperature growth ranges for select foodborne pathogens Microorganism Growth Range (oF) Salmonella sp 41.4oF – 115.2oF Clostridium botulinum A&B 50.0oF – 118.4oF Clostridium botulinum Non-proteolytic B 37.9oF – 113.0oF Clostridium botulinum E 37.9oF – 113.0oF Clostridium botulinum F 37.9oF – 113.0oF Staphylococcus aureus 44.6oF – 122.0oF Yersinia enterocolytica 29.7oF – 107.6oF Listeria monocytogenes 31.3oF – 113.0oF Vibrio cholerae 01 50.0oF – 109.4oF Vibrio parahaemolyticus 41.0oF – 111.0oF Clostridium perfringens 50.0oF – 125.6oF Bacillus cereus 39.2oF – 131.0oF Escherichia coli (pathogenic types) 44.6oF – 120.9oF Shigella spp. 43.0oF – 116.8oF Frazer, A. M., 1998: Control by Refrigeration and Freezing The infection listeriosis, can affect people of all ages, but predominantly present in those with weakened immune systems such as pregnant women, newborns and those who are elderly (CDC, 2014).