Table III 2021 Medicare National Drug Fee Schedule
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
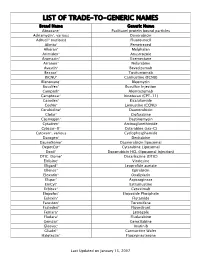
Trade-To-Generic Names
LIST OF TRADE-TO-GENERIC NAMES Brand Name Generic Name Abraxane® Paclitaxel protein bound particles Adriamycin®, various Doxorubicin Adrucil® (various) Fluorouracil Alimta® Pemetrexed Alkeran® Melphalan Arimidex® Anastrozole Aromasin® Exemestane Arranon® Nelarabine Avastin® Bevacizumab Bexxar® Tositumomab BiCNU® Carmustine (BCNU) Blenoxane® Bleomycin Busulfex® Busulfan Injection Campath® Alemtuzumab Camptosar® Irinotecan (CPT-11) Casodex® Bicalutamide CeeNu® Lomustine (CCNU) Cerubidine® Daunorubicin Clolar® Clofarabine Cosmegen® Dactinomycin Cytadren® Aminoglutethimide Cytosar-U® Cytarabine (ara-C) Cytoxan®, various Cyclophosphamide Dacogen® Decitabine DaunoXome® Daunorubicin liposomal DepotCyt® Cytarabine Liposomal Doxil® Doxorubicin HCL (liposomal injection) DTIC-Dome® Dacarbazine (DTIC) Eldisine® Vindesine Eligard® Leuprolide acetate Ellence® Epirubicin Eloxatin® Oxaliplatin Elspar® Asparaginase EmCyt® Estramustine Erbitux® Cetuximab Etopofos® Etoposide Phosphate Eulexin® Flutamide Fareston® Toremifene Faslodex® Fluvestrant Femara® Letrozole Fludara® Fludarabine Gemzar® Gemcitabine Gleevec® Imatinib Gliadel® Carmustine Wafer Halotestin® Fluoxymesterone Last Updated on January 15, 2007 Brand Name Generic Name Herceptin® Trastuzumab Hexalen® Altretamine Hycamtin® Topotecan Hydrea® Hydroxyurea Idamycin® Idarubicin Ifex® Ifosfamide Intron A® Interferon alfa-2b Iressa® Gefitinib Leukeran® Chlorambucil Leukine® Sargramostim Leustatin® Cladribine Lupron depot® Leuprolide acetate depot Lupron® Leuprolide acetate Matulane® Procarbazine Megace® -

What Are the Acute Treatments for Migraine and How Are They Used?
2. Acute Treatment CQ II-2-1 What are the acute treatments for migraine and how are they used? Recommendation The mainstay of acute treatment for migraine is pharmacotherapy. The drugs used include (1) acetaminophen, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans and (5) antiemetics. Stratified treatment according to the severity of migraine is recommended: use NSAIDs such as aspirin and naproxen for mild to moderate headache, and use triptans for moderate to severe headache, or even mild to moderate headache when NSAIDs were ineffective in the past. It is necessary to give guidance and cautions to patients having acute attacks, and explain the methods of using medications (timing, dose, frequency of use) and medication use during pregnancy and breast-feeding. Grade A Background and Objective The objective of acute treatment is to resolve the migraine attack completely and rapidly and restore the patient’s normal functions. An ideal treatment should have the following characteristics: (1) resolves pain and associated symptoms rapidly; (2) is consistently effective; (3) no recurrence; (4) no need for additional use of medication; (5) no adverse effects; (6) can be administered by the patients themselves; and (7) low cost. Literature was searched to identify acute treatments that satisfy the above conditions. Comments and Evidence The acute treatment drugs for migraine generally include (1) acetaminophens, (2) non-steroidal anti-inflammatory drugs (NSAIDs), (3) ergotamines, (4) triptans, and (5) antiemetics. For severe migraines including status migrainosus and migraine attacks refractory to treatment, (6) anesthetics, and (7) corticosteroids (dexamethasone) are used (Tables 1 and 2).1)-9) There are two approaches to the selection and sequencing of these medications: “step care” and “stratified care”. -

RESEARCH ARTICLE Lobaplatin Combined Floxuridine/Pirarubicin
DOI:http://dx.doi.org/10.7314/APJCP.2014.15.5.2057 The Effect Observation of LBP-based TACE for Unresectable Primary Hepatocellular Carcinoma RESEARCH ARTICLE Lobaplatin Combined Floxuridine/Pirarubicin-based Transcatheter Hepatic Arterial Chemoembolization for Unresectable Primary Hepatocellular Carcinoma Chang Zhao1, Xu-Jie Wang2*, Song Wang2*, Wei-Hua Feng2, Lei Shi2, Chun- Peng Yu2 Abstract Purpose: To assess the effect and safety of lobaplatin combinated floxuridine /pirarubicin in transcatheter hepatic arterial chemoembolization(TACE) of unresectable primary liver cancer. Patients and Methods: TACE combined with the chemotherapy regimen was used to treat 34 unresectable primary liver cancer patients. DSA/ MRI/CT/blood routine examinations were used to evaluate short term activity and toxicity after 4-5 weeks, the process being repeated if necessary. Results: Among the 34 cases, 1 (2.9%) showed a complete response, 21 (61.7%) a partial response, 8 (23.5%) stable disease, and 4 progressive disease, with a total effective rate of 67.6%. The content of alpha fetoprotein dropped by over 50% in 20 cases (58.8%). The rate of recovery was hepatalgia (88.2%), ascites (47.1%), appetite (55.9%), Performance Status(30.4%). The median follow-up time (MFT) was 281 days (63-558 days), and median progression-free survival was 118.5 days (95%, CI:88.8-148.2days). Adverse reactions (III-IV grade) were not common, with only 4 cases of vomiting and 2 cases of thrombocytopenia (III grade). Conclusions: Lobaplatin-based TACE is an effective and safe treatment for primary liver cancer. Keywords: Primary hepatic carcinoma - chemoembolization - platinum chemotherapy - clinical response Asian Pac J Cancer Prev, 15 (5), 2057-2060 Introduction LBP-based TACE, male 29 cases, female 5 cases, average age for 57.2±10.7 years, 12 cases were biopsy-proven, Hepatocellular carcinoma (HCC) is a common 22 cases were in line with the China Cancer Association malignant tumor . -

AHFS Pharmacologic-Therapeutic Classification System
AHFS Pharmacologic-Therapeutic Classification System Abacavir 48:24 - Mucolytic Agents - 382638 8:18.08.20 - HIV Nucleoside and Nucleotide Reverse Acitretin 84:92 - Skin and Mucous Membrane Agents, Abaloparatide 68:24.08 - Parathyroid Agents - 317036 Aclidinium Abatacept 12:08.08 - Antimuscarinics/Antispasmodics - 313022 92:36 - Disease-modifying Antirheumatic Drugs - Acrivastine 92:20 - Immunomodulatory Agents - 306003 4:08 - Second Generation Antihistamines - 394040 Abciximab 48:04.08 - Second Generation Antihistamines - 394040 20:12.18 - Platelet-aggregation Inhibitors - 395014 Acyclovir Abemaciclib 8:18.32 - Nucleosides and Nucleotides - 381045 10:00 - Antineoplastic Agents - 317058 84:04.06 - Antivirals - 381036 Abiraterone Adalimumab; -adaz 10:00 - Antineoplastic Agents - 311027 92:36 - Disease-modifying Antirheumatic Drugs - AbobotulinumtoxinA 56:92 - GI Drugs, Miscellaneous - 302046 92:20 - Immunomodulatory Agents - 302046 92:92 - Other Miscellaneous Therapeutic Agents - 12:20.92 - Skeletal Muscle Relaxants, Miscellaneous - Adapalene 84:92 - Skin and Mucous Membrane Agents, Acalabrutinib 10:00 - Antineoplastic Agents - 317059 Adefovir Acamprosate 8:18.32 - Nucleosides and Nucleotides - 302036 28:92 - Central Nervous System Agents, Adenosine 24:04.04.24 - Class IV Antiarrhythmics - 304010 Acarbose Adenovirus Vaccine Live Oral 68:20.02 - alpha-Glucosidase Inhibitors - 396015 80:12 - Vaccines - 315016 Acebutolol Ado-Trastuzumab 24:24 - beta-Adrenergic Blocking Agents - 387003 10:00 - Antineoplastic Agents - 313041 12:16.08.08 - Selective -

Novel Formulations for Treatment of Migraine
(19) TZZ _T (11) EP 2 756 756 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 23.07.2014 Bulletin 2014/30 A01N 43/42 (2006.01) A61K 31/44 (2006.01) (21) Application number: 14165112.5 (22) Date of filing: 24.04.2009 (84) Designated Contracting States: • Turanin, John AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Emeryville, CA California 94608 (US) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL • Hawley, Roger PT RO SE SI SK TR Emeryville, CA California 94608 (US) • Schuster, Jeffrey, A. (30) Priority: 28.04.2008 US 48463 Bolinas, CA California 94924 (US) (62) Document number(s) of the earlier application(s) in (74) Representative: Duxbury, Stephen et al accordance with Art. 76 EPC: Arnold & Siedsma 09739139.5 / 2 273 880 Pettenkoferstrasse 37 80336 München (DE) (71) Applicant: Zogenix, Inc. Emeryville CA 94608 (US) Remarks: This application was filed on 17-04-2014 as a (72) Inventors: divisional application to the application mentioned • Farr, Stephen J. under INID code 62. Orinda, CA California 94563 (US) (54) Novel formulations for treatment of migraine (57) Systems and methods are described for treating Systems that are self contained, portable, prefilled, and un-met medical needs in migraine and related conditions simple to self administer at the onset of a migraine attack such as cluster headache. Included are treatments that are disclosed, and preferably include a needle-free in- are both rapid onset and long acting, which include sus- jector and a high viscosity formulation, to eliminate such tained release formulations, and combination products, issues as fear of self administration with needles, and Also included are treatments for multiple symptoms of needle stick and cross contamination. -

BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, Doxorubicin, Vincristine, Cyclophosphamide
BC Cancer Protocol Summary for Treatment of Lymphoma with Dose- Adjusted Etoposide, DOXOrubicin, vinCRIStine, Cyclophosphamide, predniSONE and riTUXimab with Intrathecal Methotrexate Protocol Code LYEPOCHR Tumour Group Lymphoma Contact Physician Dr. Laurie Sehn Dr. Kerry Savage ELIGIBILITY: One of the following lymphomas: . Patients with an aggressive B-cell lymphoma and the presence of a dual translocation of MYC and BCL2 (i.e., double-hit lymphoma). Histologies may include DLBCL, transformed lymphoma, unclassifiable lymphoma, and intermediate grade lymphoma, not otherwise specified (NOS). Patients with Burkitt lymphoma, who are not candidates for CODOXM/IVACR (such as those over the age of 65 years, or with significant co-morbidities) . Primary mediastinal B-cell lymphoma Ensure patient has central line EXCLUSIONS: . Cardiac dysfunction that would preclude the use of an anthracycline. TESTS: . Baseline (required before first treatment): CBC and diff, platelets, BUN, creatinine, bilirubin. ALT, LDH, uric acid . Baseline (required, but results do not have to be available to proceed with first treatment): results must be checked before proceeding with cycle 2): HBsAg, HBcoreAb, . Baseline (optional, results do not have to be available to proceed with first treatment): HCAb, HIV . Day 1 of each cycle: CBC and diff, platelets, (and serum bilirubin if elevated at baseline; serum bilirubin does not need to be requested before each treatment, after it has returned to normal), urinalysis for microscopic hematuria (optional) . Days 2 and 5 of each cycle (or days of intrathecal treatment): CBC and diff, platelets, PTT, INR . For patients on cyclophosphamide doses greater than 2000 mg: Daily urine dipstick for blood starting on day cyclophosphamide is given. -

In Vitro and in Vivo Reversal of Resistance to 5-Fluorouracil in Colorectal Cancer Cells with a Novel Stealth Double-Liposomal Formulation
British Journal of Cancer (2007) 97, 919 – 926 & 2007 Cancer Research UK All rights reserved 0007 – 0920/07 $30.00 www.bjcancer.com In vitro and in vivo reversal of resistance to 5-fluorouracil in colorectal cancer cells with a novel stealth double-liposomal formulation 1 1 1 1 2 3 *,1 R Fanciullino , S Giacometti , C Mercier , C Aubert , C Blanquicett , P Piccerelle and J Ciccolini 1 2 EA3286-Laboratoire de Pharmacocine´tique, Universite´ de la Me´diterrane´e, Marseille, France; Department of Medicine, School of Medicine, Emory 3 University, Atlanta, GA, USA; Laboratoire de Pharmacie Gale´nique, Faculte´ de Pharmacie, 27 Bd Jean Moulin, Marseille 05 13385, France Drug resistance is a major cause of treatment failure in cancer chemotherapy, including that with the extensively prescribed antimetabolite, 5-fluorouracil (5-FU). In this study, we tried to reverse 5-FU resistance by using a double-punch strategy: combining 5-FU with a biochemical modulator to improve its tumoural activation and encapsulating both these agents in one same stealth liposome. Experiments carried out in the highly resistant, canonical SW620 human colorectal model showed a up to 80% sensitisation to 5-FU when these cells were treated with our liposomal formulation. Results with this formulation demonstrated 30% higher tumoural drug uptake, better activation with increased active metabolites including critical-5-fluoro-2-deoxyuridine-5-monophos- phate, superior inhibition (98%) of tumour thymidylate synthase, and subsequently, higher induction of both early and late apoptosis. Drug monitoring showed that higher and sustained exposure was achieved in rats treated with liposomal formulation. When examined in a xenograft animal model, our dual-agent liposomal formulation caused a 74% reduction in tumour size with a mean doubling in survival time, whereas standard 5-FU failed to exhibit significant antiproliferative activity as well as to increase the lifespan of tumour-bearing mice. -

(12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness Et Al
USOO6264,917B1 (12) United States Patent (10) Patent No.: US 6,264,917 B1 Klaveness et al. (45) Date of Patent: Jul. 24, 2001 (54) TARGETED ULTRASOUND CONTRAST 5,733,572 3/1998 Unger et al.. AGENTS 5,780,010 7/1998 Lanza et al. 5,846,517 12/1998 Unger .................................. 424/9.52 (75) Inventors: Jo Klaveness; Pál Rongved; Dagfinn 5,849,727 12/1998 Porter et al. ......................... 514/156 Lovhaug, all of Oslo (NO) 5,910,300 6/1999 Tournier et al. .................... 424/9.34 FOREIGN PATENT DOCUMENTS (73) Assignee: Nycomed Imaging AS, Oslo (NO) 2 145 SOS 4/1994 (CA). (*) Notice: Subject to any disclaimer, the term of this 19 626 530 1/1998 (DE). patent is extended or adjusted under 35 O 727 225 8/1996 (EP). U.S.C. 154(b) by 0 days. WO91/15244 10/1991 (WO). WO 93/20802 10/1993 (WO). WO 94/07539 4/1994 (WO). (21) Appl. No.: 08/958,993 WO 94/28873 12/1994 (WO). WO 94/28874 12/1994 (WO). (22) Filed: Oct. 28, 1997 WO95/03356 2/1995 (WO). WO95/03357 2/1995 (WO). Related U.S. Application Data WO95/07072 3/1995 (WO). (60) Provisional application No. 60/049.264, filed on Jun. 7, WO95/15118 6/1995 (WO). 1997, provisional application No. 60/049,265, filed on Jun. WO 96/39149 12/1996 (WO). 7, 1997, and provisional application No. 60/049.268, filed WO 96/40277 12/1996 (WO). on Jun. 7, 1997. WO 96/40285 12/1996 (WO). (30) Foreign Application Priority Data WO 96/41647 12/1996 (WO). -

Evaluation of Floxuridine Oligonucleotide Conjugates Carrying Potential Enhancers of Cellular Uptake
International Journal of Molecular Sciences Article Evaluation of Floxuridine Oligonucleotide Conjugates Carrying Potential Enhancers of Cellular Uptake Anna Aviñó 1,2,*, Anna Clua 1,2 , Maria José Bleda 1 , Ramon Eritja 1,2,* and Carme Fàbrega 1,2 1 Institute for Advanced Chemistry of Catalonia (IQAC-CSIC), Jordi Girona 18-26, E-08034 Barcelona, Spain; [email protected] (A.C.); [email protected] (M.J.B.); [email protected] (C.F.) 2 Networking Center on Bioengineering, Biomaterials and Nanomedicine (CIBER-BBN), Jordi Girona 18-26, E-08034 Barcelona, Spain * Correspondence: [email protected] (A.A.); [email protected] (R.E.); Tel.: +34-934-006-100 (A.A.) Abstract: Conjugation of small molecules such as lipids or receptor ligands to anti-cancer drugs has been used to improve their pharmacological properties. In this work, we studied the biological effects of several small-molecule enhancers into a short oligonucleotide made of five floxuridine units. Specifically, we studied adding cholesterol, palmitic acid, polyethyleneglycol (PEG 1000), folic acid and triantennary N-acetylgalactosamine (GalNAc) as potential enhancers of cellular uptake. As expected, all these molecules increased the internalization efficiency with different degrees depending on the cell line. The conjugates showed antiproliferative activity due to their metabolic activation by nuclease degradation generating floxuridine monophosphate. The cytotoxicity and apoptosis assays showed an increase in the anti-cancer activity of the conjugates related to the floxuridine oligomer, but this effect did not correlate with the internalization results. Palmitic and folic acid conjugates provide the highest antiproliferative activity without having the highest internalization Citation: Aviñó, A.; Clua, A.; Bleda, results. -

Parkinson's Disease Fact Sheet
Parkinson’s Disease Fact Sheet About Parkinson’s Disease Parkinson’s disease is a progressive, incurable neurological disorder associated with a loss of dopamine-generating cells in the brain. It is primarily associated with progressive loss of motor control, but it results in a complex array of symptoms, including many non-motor symptoms. Parkinson’s impacts an estimated one million people in the United States. Critical Clinical Care Considerations • To avoid serious side effects, Parkinson’s patients need their medications on time, every time — do not skip or postpone doses. • Write down the exact times of day medications are to be administered so that doses are given on the same schedule the patient follows at home. • Do not substitute Parkinson’s medications or stop levodopa therapy abruptly. • Resume medications immediately following procedures, unless vomiting or severely incapacitated. • If an antipsychotic is necessary, use pimavanserin (Nuplazid), quetiapine (Seroquel) or clozapine (Clozaril). • Be alert for symptoms of dysphagia (trouble swallowing) and risk of pneumonia. • Ambulate as soon as medically safe. Patients may require assistance. Common Symptoms of Parkinson’s Disease Motor Non-Motor • Shaking or tremor at rest • Depression • Bradykinesia or freezing (being stuck • Anxiety in place when attempting to walk) • Constipation • Low voice volume or muffled speech • Cognitive decline and dementia • Lack of facial expression • Impulse control disorders • Stiffness or rigidity of the arms, legs • Orthostatic hypotension or -

Pharmacotherapy of Impaired Mucociliary Clearance in Non-CF Pediatric Lung Disease
Pediatric Pulmonology 42:989–1001 (2007) State of the Art Pharmacotherapy of Impaired Mucociliary Clearance in Non-CF Pediatric Lung Disease. A Review of the Literature 1 1 1,2 Ruben Boogaard, MD, * Johan C. de Jongste, MD, PhD, and Peter J.F.M. Merkus, MD, PhD Summary. Mucoactive agents are used to treat a variety of lung diseases involving impaired mucociliary clearance or mucus hypersecretion. The mucoactive agents studied most frequently are N-acetylcysteine (NAC), recombinant human DNase (rhDNase), and hypertonic saline. Studies on the efficacy of these have been mainly conducted in adults, and in patients with cystic fibrosis (CF). The exact role of mucoactive agents in children with non-CF lung disease is not well established. We present an overview of the current literature reporting clinical outcome measures of treatment with NAC, rhDNase, and hypertonic saline in children. Pediatr Pulmonol. 2007; 42:989–1001. ß 2007 Wiley-Liss, Inc. Key words: mucolytic; sulfhydryl compounds; N-acetylcysteine; dornase alfa; hyper- tonic saline; respiratory tract disease. INTRODUCTION One possible means to evaluate a mucoactive agent is to assess its effect on mucociliary clearance (MCC) or cough Mucus clearance is an important primary innate airway clearance with the use of radiolabeled aerosol. Discussing defense mechanism, and our understanding of the key this subject is outside the scope of this review. Moreover, parameters underlying its function has grown rapidly in the studies on mucoactive agents in CF patients, and studies last decade.1,2 Impaired mucus clearance or mucus hyper- on physiotherapy or secretion clearance techniques in secretion are important clinical features in diseases such as (pediatric) lung disease patients have been reviewed by cystic fibrosis (CF), recurrent bronchitis, asthma, and others, and will therefore not be discussed in this review. -

Supportive Care Medications
th Clinical Pharmacy Guide: Cancer Drug Treatment Assessment and Review 5 Edition Supportive Care Medications Contents Introduction ................................................................................................................. 2 Supportive Care Information in Protocols .................................................................... 2 Antidiarrheals .............................................................................................................. 5 Loperamide ............................................................................................................. 5 Antiemetics .................................................................................................................. 6 Emetogenicity .......................................................................................................... 7 Types of Chemotherapy-Induced Nausea and Vomiting .......................................... 7 Classifications of Antiemetics .................................................................................. 8 Anti-infective Agents .................................................................................................. 10 Antibiotics .............................................................................................................. 10 Antivirals ................................................................................................................ 11 Arthralgias/Myalgias .................................................................................................