Repeated Die Off of Endangered Fish in Upper Klamath Lake
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Trout Fishing in Hillsdale July, 2012 Guy Winig, Member Hillsdale Conservation Advisory Council
Roe Jan Brown-released to fight another day. Trout Fishing in Hillsdale July, 2012 Guy Winig, member Hillsdale Conservation Advisory Council Trout are our canaries in a coalmine. They are an excellent indicator of stream health, requiring clean, cold, well-oxygenated water to thrive. Without very high quality surface waters, trout just cannot survive. Hillsdale is fortunate to have several miles of trout streams including the Roeliff Jansen Kill, Taconic Creek, Agawamuck Creek and Green River with numerous smaller and unnamed tributaries contributing to them. Our streams and their residents endure many perils including natural flood and drought cycles as well as man made impacts like pollution, invasive species, barriers and over –fishing. Erosion adds silt that smothers aquatic food sources and gravel spawning beds. Introduced Didymo algae (a.k.a. ‘Rock Snot’) now found in the Green River, also chokes the streambed. Loss of vegetation exposes water to heating and eliminates important breeding, feeding and cover areas for trout. Readers may recall the massive fish kill that resulted from the release of manure into the Roe Jan in the 90’s or tales of unscrupulous ‘sportsmen’ using small explosives and chlorine to kill all the fish in a segment of stream. Maybe the most harmful and insidious are non-point sources of pollution such as chemicals, fertilizers and heated runoff from parking lots, roads, roofs, crop fields and lawns. In 2009 and 2010, members of the community with help from the Columbia-Greene chapter of Trout Unlimited and the N.Y.S.D.E.C. Trees for Tributaries program planted several hundred small trees and shrubs on the banks and flood plain of the Roe Jan Kill through the Roe Jan Park in an effort to stabilize banks, introduce vegetative cover and provide shade to maintain cool stream temperatures. -

Localized Plankton Blooms and Jubilees on the Gulf Coast Gordon Gunter Gulf Coast Research Laboratory
Gulf Research Reports Volume 6 | Issue 3 January 1979 Localized Plankton Blooms and Jubilees on the Gulf Coast Gordon Gunter Gulf Coast Research Laboratory Charles H. Lyles Gulf Coast Research Laboratory DOI: 10.18785/grr.0603.12 Follow this and additional works at: http://aquila.usm.edu/gcr Part of the Marine Biology Commons Recommended Citation Gunter, G. and C. H. Lyles. 1979. Localized Plankton Blooms and Jubilees on the Gulf Coast. Gulf Research Reports 6 (3): 297-299. Retrieved from http://aquila.usm.edu/gcr/vol6/iss3/12 This Short Communication is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf and Caribbean Research by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Gulf Research Reports, Vol. 6,NO. 3,291-299, 1919. LOCALIZED PLANKTON BLOOMS AND JUBILEES ON THE GULF COAST GORDON GUNTER AND CHARLES H. LYLES Gulf Coast Research Laboratory, Ocean Springs, Mississippi 39564 and Gulf States Marine Fisheries Commission, Ocean Springs, Mississippi 39564 ABSTRACT The writers describe various small types of plankton blooms such as those occurring in boat slips, the head of a large bayou and a strip type bloom of Chaeroceras on the Gulf beach. Oyster kills from “poison water” draining off of marshes are said to be caused by plankton bloom. Small “jubilees” are said to be caused by localized blooms and one of these is described as it occurred. In November 1938, Dr. Margaretha Brongersma-Sanders of billions of Chaetoceras sp., a common open sea diatom. -

Characterizing Tribal Cultural Landscapes, Volume II: Tribal Case
OCS Study BOEM 2017-001 Characterizing Tribal Cultural Landscapes Volume II: Tribal Case Studies US Department of the Interior Bureau of Ocean Energy Management Pacific OCS Region This page intentionally left blank. OCS Study BOEM 2017-001 Characterizing Tribal Cultural Landscapes Volume II: Tribal Case Studies David Ball Rosie Clayburn Roberta Cordero Briece Edwards Valerie Grussing Janine Ledford Robert McConnell Rebekah Monette Robert Steelquist Eirik Thorsgard Jon Townsend Prepared under BOEM-NOAA Interagency Agreement M12PG00035 by National Oceanic and Atmospheric Administration Office of National Marine Sanctuaries 1305 East-West Highway, SSMC4 Silver Spring, MD 20910 Makah Tribe Confederated Tribes of Grand Ronde Community of Oregon Yurok Tribe National Marine Sanctuary Foundation US Department of Commerce National Oceanic and Atmospheric Administration Office of National Marine Sanctuaries US Department of the Interior Bureau of Ocean Energy Management Pacific OCS Region December 31, 2017 This page intentionally left blank. DISCLAIMER This study was funded, in part, by the US Department of the Interior, Bureau of Ocean Energy Management (BOEM), Pacific Outer Continental Shelf (OCS) Region, Camarillo, CA, through Interagency Agreement Number M12PG00035 with the US Department of Commerce, National Oceanic and Atmospheric Administration (NOAA). This report has been technically reviewed by BOEM and it has been approved for publication. The views and conclusions contained in this document are those of the authors and should not be interpreted as representing the opinions or policies of the US Government, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. REPORT AVAILABILITY This report can be downloaded from the Bureau of Ocean Energy Management’s Recently Completed Environmental Studies – Pacific webpage at https://www.boem.gov/Pacific-Completed-Studies/. -

Literature Based Characterization of Resident Fish Entrainment-Turbine
Draft Technical Memorandum Literature Based Characterization of Resident Fish Entrainment and Turbine-Induced Mortality Klamath Hydroelectric Project (FERC No. 2082) Prepared for PacifiCorp Prepared by CH2M HILL September 2003 Contents Introduction...................................................................................................................................1 Objectives ......................................................................................................................................1 Study Approach ............................................................................................................................2 Fish Entrainment ..............................................................................................................2 Turbine-induced Mortality .............................................................................................2 Characterization of Fish Entrainment ......................................................................................2 Magnitude of Annual Entrainment ...............................................................................9 Size Composition............................................................................................................10 Species Composition ......................................................................................................10 Seasonal and Diurnal Distribution...............................................................................15 Turbine Mortality.......................................................................................................................18 -

Water Allocation in the Klamath Reclamation Project (Oregon State
Oregon State University Extension Service Special Report 1037 December 2002 Water Allocation in the Klamath Reclamation Project, 2001: An Assessment of Natural Resource, Economic, Social, and Institutional Issues with a Focus on the Upper Klamath Basin William S. Braunworth, Jr. Assistant Extension Agriculture Program Leader Oregon State University Teresa Welch Publications Editor Oregon State University Ron Hathaway Extension agriculture faculty, Klamath County Oregon State University Authors William Boggess, department head, Department of William K. Jaeger, associate professor of agricul- Agricultural and Resource Economics, Oregon tural and resource economics and Extension State University agricultural and resource policy specialist, Oregon State University William S. Braunworth, Jr., assistant Extension agricultural program leader, Oregon State Robert L. Jarvis, professor of fisheries and University wildlife, Oregon State University Susan Burke, researcher, Department of Agricul- Denise Lach, codirector, Center for Water and tural and Resource Economics, Oregon State Environmental Sustainability, Oregon State University University Harry L. Carlson, superintendent/farm advisor, Kerry Locke, Extension agriculture faculty, University of California Intermountain Research Klamath County, Oregon State University and Extension Center Jeff Manning, graduate student, Department of Patty Case, Extension family and community Fisheries and Wildlife, Oregon State University development faculty, Klamath County, Oregon Reed Marbut, Oregon Water Resources -

Water-Quality Data from Upper Klamath and Agency Lakes, Oregon, 2009–10
Prepared in cooperation with the Bureau of Reclamation Water-Quality Data from Upper Klamath and Agency Lakes, Oregon, 2009–10 Open-File Report 2012–1142 U.S. Department of the Interior U.S. Geological Survey Cover: Meteorological and water quality monitoring site MDN on Upper Klamath Lake, Oregon, with Mt. McLoughlin in the background. (Photograph by D. Blake Eldridge, U.S. Geological Survey, July 12, 2011.) Water-Quality Data from Upper Klamath and Agency Lakes, Oregon, 2009–10 By D. Blake Eldridge, Sara L. Caldwell Eldridge, Liam N. Schenk, Dwight Q. Tanner, and Tamara M. Wood Prepared in cooperation with the Bureau of Reclamation Open-File Report 2012–1142 U.S. Department of the Interior U.S. Geological Survey U.S. Department of the Interior KEN SALAZAR, Secretary U.S. Geological Survey Marcia K. McNutt, Director U.S. Geological Survey, Reston, Virginia: 2012 For more information on the USGS—the Federal source for science about the Earth, its natural and living resources, natural hazards, and the environment, visit http://www.usgs.gov or call 1-888-ASK-USGS. For an overview of USGS information products, including maps, imagery, and publications, visit http://www.usgs.gov/pubprod To order this and other USGS information products, visit http://store.usgs.gov Suggested citation: Eldridge. D.B., Caldwell Eldridge, S.L., Schenk, L.N., Tanner, D.Q., and Wood, T.M., 2012, Water-quality data from Upper Klamath and Agency Lakes, Oregon, 2009–10: U.S. Geological Survey Open-File Report 2012–1142, 32 p. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. -

Ecology of Upper Klamath Lake Shortnose and Lost River Suckers
ECOLOGY OF UPPER KLAMATH LAKE SHORTNOSE AND LOST RIVER SUCKERS 4. The Klamath Basin sucker species complex 1999 ANNUAL REPORT (partial) SUBMITTED TO U. S. Biological Resources Division US Geological Survey 104 Nash Hall Oregon State University Corvallis, Oregon 97331-3803 & Klamath Project U. S. Bureau of Reclamation 6600 Washburn Way Klamath Falls, OR 97603 by Douglas F. ~arkle', Martin R. ~avalluzzi~,Thomas E. owli in^^ and David .Simon1 1Oregon Cooperative Research Unit 104 Nash Hall Department of Fisheries and Wildlife Oregon State University Corvallis, Oregon 97331-3803 E -mai1 : douglas.markle@,orst.edu 2Department of Biology Arizona State University Tempe, AZ 85287-1501 Phone: 480-965-1626 Fax: 480-965-2519 E -mai 1 : [email protected] July 26, 2000 There are 13 genera and 68 species of catostomids (Nelson 1994) with three genera and four species occurring in Klamath Basin (Bond 1994)- Catostomus rimiculus Gilbert and Snyder, 1898 (Klamath smallscale sucker, KSS), C. snyderi Gilbert 1898 (Klamath largescale sucker, KLS), Chasmistes brevirostris Cope, 1879 (shortnose sucker, SNS), and Deltistes luxatus (Cope, 1879) (Lost River sucker, LRS). Lost River and shortnose suckers are federally listed endangered species (U.S. Fish and Wildlife Service 1988). The four Klamath Basin suckers are similar in overall body shape, but highly variable, and are distinguished by feeding-related structures, adult habitat and geography. The two Catostomus species have large lips, widely-spaced gillrakers, and are primarily river dwellers with C. snyderi mostly found in the upper basin and C. rimiculus in the lower basin and adjacent Rogue River. Deltistes luxatus has smaller lips, short "deltoid" Catostomus-like gillrakers, and is primariliy a lake dweller. -

Dispersal of Larval Suckers at the Williamson River Delta, Upper Klamath Lake, Oregon, 2006–09
Prepared in cooperation with the Bureau of Reclamation Dispersal of Larval Suckers at the Williamson River Delta, Upper Klamath Lake, Oregon, 2006–09 Scientific Investigations Report 2012–5016 U.S. Department of the Interior U.S. Geological Survey Cover: Inset: Larval sucker from Upper Klamath Lake, Oregon. (Photograph taken by Allison Estergard, Student, Oregon State University, Corvallis, Oregon, 2011.) Top: Photograph taken from the air of the flooded Williamson River Delta, Upper Klamath Lake, Oregon. (Photograph taken by Charles Erdman, Fisheries Technician, Williamson River Delta Preserve, Klamath Falls, Oregon, 2008.) Bottom left: Photograph of a pop net used by The Nature Conservancy to collect larval suckers in Upper Klamath Lake and the Williamson River Delta, Oregon. (Photograph taken by Heather Hendrixson, Director, Williamson River Delta Preserve, Klamath Falls, Oregon, 2006.) Bottom middle: Photograph of a larval trawl used by Oregon State University to collect larval suckers in Upper Klamath Lake and the Williamson River Delta, Oregon. (Photograph taken by David Simon, Senior Faculty Research Assistant, Oregon State University, Corvallis, Oregon, 2010.) Bottom right: Photograph of a plankton net used by the U.S. Geological Survey to collect larval suckers in Upper Klamath Lake and the Williamson River Delta, Oregon. (Photographer unknown, Klamath Falls, Oregon, 2009.) Dispersal of Larval Suckers at the Williamson River Delta, Upper Klamath Lake, Oregon, 2006–09 By Tamara M. Wood, U.S. Geological Survey, Heather A. Hendrixson, The Nature Conservancy, Douglas F. Markle, Oregon State University, Charles S. Erdman, The Nature Conservancy, Summer M. Burdick, U.S. Geological Survey, Craig M. Ellsworth, U.S. Geological Survey, and Norman L. -

Yurok Final Brief
Case 3:16-cv-06863-WHO Document 107 Filed 03/23/18 Page 1 of 22 JEFFREY H. WOOD, Acting Assistant Attorney General 1 Environment & Natural Resources Division 2 SETH M. BARSKY, Chief S. JAY GOVINDAN, Assistant Chief 3 ROBERT P. WILLIAMS, Sr. Trial Attorney KAITLYN POIRIER, Trial Attorney 4 U.S. Department of Justice 5 Environment & Natural Resources Division Wildlife & Marine Resources Section 6 Ben Franklin Station, P.O. Box 7611 7 Washington, D.C. 20044-7611 Tel: 202-307-6623; Fax: 202-305-0275 8 Email: [email protected] Email: [email protected] 9 10 Attorneys for Federal Defendants 11 UNITED STATES DISTRICT COURT 12 FOR THE NORTHERN DISTRICT OF CALIFORNIA 13 SAN FRANCISCO DIVISION 14 YUROK TRIBE, et al., ) 15 Case No. 3:16-cv-06863-WHO ) 16 Plaintiff, ) ) 17 FEDERAL DEFENDANTS’ RESPONSE v. ) TO DEFENDANT-INTERVENORS’ 18 ) MOTION FOR RELIEF FROM U.S. BUREAU OF RECLAMATION, et al., ) JUDGMENT AND/OR STAY OF 19 ) ENFORCEMENT (ECF No. 101) Defendants, ) 20 ) 21 and ) ) 22 KLAMATH WATER USERS ) ASSOCIATION, et al., ) 23 ) 24 Defendant-Intervenors. ) 25 26 27 28 1 Federal Defendants’ Response to Intervenors’ Motion for Relief 3:16-cv-6863-WHO Case 3:16-cv-06863-WHO Document 107 Filed 03/23/18 Page 2 of 22 1 TABLE OF CONTENTS 2 I. INTRODUCTION 3 3 II. FACTUAL BACKGROUND 5 4 A. Hydrologic Conditions In Water Year 2018 5 5 B. 2013 Biological Opinion Requirements for Suckers 5 6 III. DISCUSSION 7 7 A. Given Hydrologic Conditions, Guidance Measures 1 8 and 4 Cannot Both Be Implemented As They Were Designed Without Impermissibly Interfering With 9 Conditions Necessary to Protect Endangered Suckers 7 10 1. -

FISHING NEWSLETTER 2020/2021 Table of Contents FWP Administrative Regions and Hatchery Locations
FISHING NEWSLETTER 2020/2021 Table of Contents FWP Administrative Regions and Hatchery Locations .........................................................................................3 Region 1 Reports: Northwest Montana ..........................................................................................................5 Region 2 Reports: West Central Montana .....................................................................................................17 Region 3 Reports: Southwest Montana ........................................................................................................34 Region 4 Reports: North Central Montana ...................................................................................................44 Region 5 Reports: South Central Montana ...................................................................................................65 Region 6 Reports: Northeast Montana ........................................................................................................73 Region 7 Reports: Southeast Montana .........................................................................................................86 Montana Fish Hatchery Reports: .......................................................................................................................92 Murray Springs Trout Hatchery ...................................................................................................................92 Washoe Park Trout Hatchery .......................................................................................................................93 -
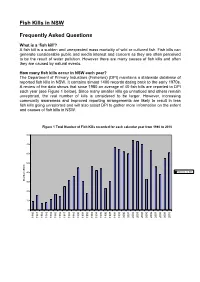
Fish Kills in NSW Frequently Asked Questions
Fish Kills in NSW Frequently Asked Questions What is a ‘fish kill’? A fish kill is a sudden and unexpected mass mortality of wild or cultured fish. Fish kills can generate considerable public and media interest and concern as they are often perceived to be the result of water pollution. However there are many causes of fish kills and often they are caused by natural events. How many fish kills occur in NSW each year? The Department of Primary Industries (Fisheries) (DPI) maintains a statewide database of reported fish kills in NSW. It contains almost 1400 records dating back to the early 1970s. A review of the data shows that since 1980 an average of 40 fish kills are reported to DPI each year (see Figure 1 below). Since many smaller kills go unnoticed and others remain unreported, the real number of kills is considered to be larger. However, increasing community awareness and improved reporting arrangements are likely to result in less fish kills going unreported and will also assist DPI to gather more information on the extent and causes of fish kills in NSW. Figure 1 Total Number of Fish Kills recorded for each calendar year from 1980 to 2010 80 70 60 50 40 Total no. of kills Number of kills 30 20 10 0 1980 1981 1982 1983 1984 1985 1986 1987 1988 1989 1990 1991 1992 1993 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010 What species of fish are affected? Over 100 species of fish, including finfish, molluscs and crustaceans, have been reported in fish kills in NSW since records began. -

Distribution of Anadromomous Fishes in the Upper Klamath River
Distribution of Anadromomous Fishes in the Upper Klamath River Watershed Prior to Hydropower Dams— A Synthesis of the Historical Evidence fisheries history Knowledge of the historical distribution of anadromous fish is important to guide man- agement decisions in the Klamath River including ongoing restoration and regional recovery of coho salmon (Oncorhynchus kisutch). Using various sources, we determined the historical distribution of anadromous fish above Iron Gate Dam. Evidence for the feature largest, most utilized species, Chinook salmon (Oncorhynchus tshawytscha), was avail- ABSTRACT able from multiple sources and clearly showed that this species historically migrated upstream into tributaries of Upper Klamath Lake. Available information indicates that the distribution of steelhead (Oncorhynchus mykiss) extended to the Klamath Upper Basin as well. Coho salmon and anadromous lamprey (Lampetra tridentata) likely were distributed upstream at least to the vicinity of Spencer Creek. A population of anadro- mous sockeye salmon (Oncorhynchus nerka) may have occurred historically above Iron Gate Dam. Green sturgeon (Acipenser medirostris), chum salmon (Oncorhynchus keta), pink salmon (Oncorhynchus gorbuscha), coastal cutthroat trout (Oncorhynchus clarki clarki), and eulachon (Thaleichthys pacificus) were restricted to the Klamath River well below Iron Gate Dam. This synthesis of available sources regarding the historical extent of these species’ upstream distribution provides key information necessary to guide management and habitat restoration efforts. Introduction John B. Hamilton Gary L. Curtis Gatschet’s statement is that salmon ascend the Klamath river twice a year, in June and again in autumn. This is in agreement with my information, that the run comes in the middlefinger Scott M. Snedaker month [sic], May–June, and that the large fish run in the fall...They ascend all the rivers David K.