University of Michigan University Library
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ostracoda an Introduction.Pdf
The Ostracoda (from Wikipedia, 5/5/2009: http://en.wikipedia.org/wiki/Ostracod) Ostracoda is a class of the Crustacea, sometimes known as the seed shrimp because of their appearance. Ostracods are small crustaceans, typically around one mm in size, but varying between 0.2 to 30 mm, laterally compressed and protected by a bivalve-like, chitinous or calcareous valve or "shell". The hinge of the two valves is in the upper, dorsal region of the body. Some 65,000 species (13,000 of which are extant taxa) have been identified, grouped into several orders. This group may not be monophyletic. Ostracod taxa are grouped into a Class based on gross morphology. Ecologically, marine ostracods can be part of the zooplankton or (most commonly) they are part of the benthos, living on or inside the upper layer of the sea floor. Many ostracods, especially the Podocopida, are also found in fresh water and some are known from humid continental forest soils. The body consists of a cephalon (head), separated from the thorax by a slight constriction. The segmentation is unclear. The abdomen is regressed or absent whereas the adult gonads are relatively large. There are 5–8 pairs of appendages. The branchial plates are responsible for oxygenation. The epidermal cells may also secrete calcium carbonate after the chitinous layer is formed, resulting in a chalk layer enveloped by chitin. This calcification is not equally pronounced in all orders. During every instar transition, the old carapace (chitinous and calcified) is rejected and a new, larger is formed and calcified. The outer lamella calcifies completely, while the inner lamella calcifies partially, with the rest remaining chitinous. -
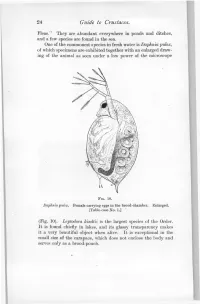
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

15 International Symposium on Ostracoda
Berliner paläobiologische Abhandlungen 1-160 6 Berlin 2005 15th International Symposium on Ostracoda In Memory of Friedrich-Franz Helmdach (1935-1994) Freie Universität Berlin September 12-15, 2005 Abstract Volume (edited by Rolf Kohring and Benjamin Sames) 2 ---------------------------------------------------------------------------------------------------------------------------------------- Preface The 15th International Symposium on Ostracoda takes place in Berlin in September 2005, hosted by the Institute of Geological Sciences of the Freie Universität Berlin. This is the second time that the International Symposium on Ostracoda has been held in Germany, following the 5th International Symposium in Hamburg in 1974. The relative importance of Ostracodology - the science that studies Ostracoda - in Germany is further highlighted by well-known names such as G.W. Müller, Klie, Triebel and Helmdach, and others who stand for the long tradition of research on Ostracoda in Germany. During our symposium in Berlin more than 150 participants from 36 countries will meet to discuss all aspects of living and fossil Ostracoda. We hope that the scientific communities working on the biology and palaeontology of Ostracoda will benefit from interesting talks and inspiring discussions - in accordance with the symposium's theme: Ostracodology - linking bio- and geosciences We wish every participant a successful symposium and a pleasant stay in Berlin Berlin, July 27th 2005 Michael Schudack and Steffen Mischke CONTENT Schudack, M. and Mischke, S.: Preface -

Taxonomy of Quaternary Deep-Sea Ostracods from the Western North Atlantic Ocean
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2009 Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean Moriaki Yasuhara National Museum of Natural History, Smithsonian Institution, [email protected] Hisayo Okahashi National Museum of Natural History, Smithsonian Institution, [email protected] Thomas M. Cronin U.S. Geological Survey, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Earth Sciences Commons Yasuhara, Moriaki; Okahashi, Hisayo; and Cronin, Thomas M., "Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean" (2009). USGS Staff -- Published Research. 242. https://digitalcommons.unl.edu/usgsstaffpub/242 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. [Palaeontology, Vol. 52, Part 4, 2009, pp. 879–931] TAXONOMY OF QUATERNARY DEEP-SEA OSTRACODS FROM THE WESTERN NORTH ATLANTIC OCEAN by MORIAKI YASUHARA*, HISAYO OKAHASHI* and THOMAS M. CRONIN *Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, MRC 121, PO Box 37012, Washington, DC 20013-7012, USA; e-mails: [email protected] or [email protected] (M.Y.), [email protected] (H.O.) U.S. Geological Survey, -

Ostracoda and Foraminifera from Paleocene (Olinda Well), Paraíba Basin, Brazilian Northeast
Anais da Academia Brasileira de Ciências (2017) 89(3): 1443-1463 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201720160768 www.scielo.br/aabc | www.fb.com/aabcjournal Ostracoda and foraminifera from Paleocene (Olinda well), Paraíba Basin, Brazilian Northeast ENELISE K. PIOVESAN¹, ROBBYSON M. MELO¹, FERNANDO M. LOPES², GERSON FAUTH³ and DENIZE S. COSTA³ ¹Laboratório de Geologia Sedimentar e Ambiental/LAGESE, Universidade Federal de Pernambuco, Departamento de Geologia, Centro de Tecnologia e Geociências, Av. Acadêmico Hélio Ramos, s/n, 50740-530 Recife, PE, Brazil ²Instituto Tecnológico de Micropaleontologia/itt Fossil, Universidade do Vale do Rio dos Sinos/UNISINOS, Av. Unisinos, 950, 93022-750 São Leopoldo, RS, Brazil ³PETROBRAS/CENPES/PDEP/BPA, Rua Horácio Macedo, 950, Cidade Universitária, Ilha do Fundão, Prédio 32, 21941-915 Rio de Janeiro, RJ, Brazil Manuscript received on November 7, 2016; accepted for publication on March 16, 2017 ABSTRACT Paleocene ostracods and planktonic foraminifera from the Maria Farinha Formation, Paraíba Basin, are herein presented. Eleven ostracod species were identified in the genera Cytherella Jones, Cytherelloidea Alexander, Eocytheropteron Alexander, Semicytherura Wagner, Paracosta Siddiqui, Buntonia Howe, Soudanella Apostolescu, Leguminocythereis Howe and, probably, Pataviella Liebau. The planktonic foraminifera are represented by the genera Guembelitria Cushman, Parvularugoglobigerina Hofker, Woodringina Loeblich and Tappan, Heterohelix Ehrenberg, Zeauvigerina Finlay, Muricohedbergella Huber and Leckie, and Praemurica Olsson, Hemleben, Berggren and Liu. The ostracods and foraminifera analyzed indicate an inner shelf paleoenvironment for the studied section. Blooms of Guembelitria spp., which indicate either shallow environments or upwelling zones, were also recorded reinforcing previous paleoenvironmental interpretations based on other fossil groups for this basin. -

Data Report: Pliocene and Pleistocene Deep-Sea Ostracods from Integrated
Tada, R., Murray, R.W., Alvarez Zarikian, C.A., and the Expedition 346 Scientists Proceedings of the Integrated Ocean Drilling Program, Volume 346 Data report: Pliocene and Pleistocene deep-sea ostracods from Integrated Ocean Drilling Program Site U1426 (Expedition 346)1 Katsura Yamada,2 Kentaro Kuroki,3 and Tatsuhiko Yamaguchi4 Chapter contents Abstract Abstract . 1 Little is known about fossil ostracods from deep-sea drilling sites in the marginal sea bordered by the Eurasian continent, the Ko- Introduction . 1 rean Peninsula, and the Japanese Islands. This report presents tax- Site location and setting . 2 onomic descriptions for the Pliocene and early Pleistocene deep- Material and methods. 2 sea ostracods at Integrated Ocean Drilling Program Expedition Results . 2 346 Site U1426 on the Oki Ridge in the marginal sea. We found Acknowledgments. 2 23 species and 12 genera in discrete core samples. The ostracods References . 2 constitute taxa that dwell under the Tsushima Warm Current and Figure. 7 the Japan Sea Intermediate-Proper Water. We present taxonomic Tables. 8 summaries of ostracod species distribution and ecology in addi- Plates . 15 tion to scanning electron microscopic images. Taxonomic notes . 18 Introduction Previously, Pliocene and Pleistocene ostracods from the marginal sea were investigated using specimens from mainly outcrops ex- posed in the marginal sea side of the Japanese Islands. Most of these studies examined shallow-marine ostracods from the Setana (Hayashi, 1988), Omma (Ozawa and Kamiya, 2001, 2005), Yabuta (Cronin et al., 1994), Mita (Goto et al., 2014b), Shichiba (Ozawa, 2010; Ishida et al., 2012), and Sasaoka (Irizuki, 1989; Yamada et al., 2002) formations. -

Cypris 2016-2017
CYPRIS 2016-2017 Illustrations courtesy of David Siveter For the upper image of the Silurian pentastomid crustacean Invavita piratica on the ostracod Nymphateline gravida Siveter et al., 2007. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, and M.D. Sutton. 2015. A 425-million-year- old Silurian pentastomid parasitic on ostracods. Current Biology 23: 1-6. For the lower image of the Silurian ostracod Pauline avibella Siveter et al., 2012. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, M.D. Sutton, and S.C. Joomun. 2013. A Silurian myodocope with preserved soft-parts: cautioning the interpretation of the shell-based ostracod record. Proceedings of the Royal Society London B, 280 20122664. DOI:10.1098/rspb.2012.2664 (published online 12 December 2012). Watermark courtesy of Carin Shinn. Table of Contents List of Correspondents Research Activities Algeria Argentina Australia Austria Belgium Brazil China Czech Republic Estonia France Germany Iceland Israel Italy Japan Luxembourg New Zealand Romania Russia Serbia Singapore Slovakia Slovenia Spain Switzerland Thailand Tunisia United Kingdom United States Meetings Requests Special Publications Research Notes Photographs and Drawings Techniques and Methods Awards New Taxa Funding Opportunities Obituaries Horst Blumenstengel Richard Forester Franz Goerlich Roger Kaesler Eugen Kempf Louis Kornicker Henri Oertli Iraja Damiani Pinto Evgenii Schornikov Michael Schudack Ian Slipper Robin Whatley Papers and Abstracts (2015-2007) 2016 2017 In press Addresses Figure courtesy of Francesco Versino, -

The Taxonomy and Biogeography of Macrofaunal Ostracod Crustaceans
The taxonomy and biogeography of macrofaunal ostracod crustaceans, with focus on the abyssal benthic Pacific fauna relevant to the CCFZ Ivana Karanovic Hanyang University, Department of Life Science, College of Natural Sciences, Seoul 133-791, Korea University of Tasmania, IMAS, Hobart, TAS, 7001, Australia e-mail: [email protected] Few words about myself Italy(Salerno, 2 years) Serbia (Novi Sad, born) Australia (Perth & Hobart, 10 years) Germany (Hamburg, 2 years) South Korea (Seoul, 3.5 years) • Started working on ostracods 15 years ago • Worked on faunas from all continents (including Antarctica) • and from all environments: from freshwater puddles to deep sea • I don’t particularly like ostracods • I like the fact that ostracods give insight into many aspects of biology General information on ostracods • Named in 1802 by Latreille • Name comes from the Greek óstrakon, meaning shell or tile • Common name in English: “mussel shrimp” or “seed shrimp” • In German it is “Muschelkrebse” • Live in all aquatic habitats on the planet Fossil record Systematics • Previously in the class Maxillopoda • Currently recognized as one of the 7 classes of the phylum Crustacea Currently divided into two subclasses 1. Myodocopa 2. Podocopa Systematics cont. 4a. 1. Subclass Myodocopa 1. Order Myodocopina 2. Order Halocyprida 4b. a) Suborder Halocypridina b) Suborder Cladocopina 2a. Subclass Podocopa 3. Order Platycopida 4. Order Podocopida 4c. a) Suborder Bairdiocopina b) Suborder Cytherocopina 2b. c) Suborder Darwinulocopina d) Suborder Cypridocopina 4d. e) Suborder Sigilliocopina 3. Photo credits: 4e. 1, 2: S.N. Brandao 3: Brandao & Yasuhara 4b, c: D. Keyser 4e: From Maddocks (1972) Morphology a. -

Crustacea: Ostracoda) De Pozas Temporales
Heterocypris bosniaca (Petkowski et al., 2000): Ecología y ontogenia de un ostrácodo (Crustacea: Ostracoda) de pozas temporales. ESIS OCTORAL T D Josep Antoni Aguilar Alberola Departament de Microbiologia i Ecologia Universitat de València Programa de doctorat en Biodiversitat i Biologia Evolutiva Heterocypris bosniaca (Petkowski et al., 2000): Ecología y ontogenia de un ostrácodo (Crustacea: Ostracoda) de pozas temporales. Tesis doctoral presentada por Josep Antoni Aguilar Alberola 2013 Dirigida por Francesc Mesquita Joanes Imagen de cubierta: Vista lateral de la fase eclosionadora de Heterocypris bosniaca. Más detalles en el capítulo V. Tesis titulada "Heterocypris bosniaca (Petkowski et al., 2000): Ecología y ontogenia de un ostrácodo (Crustacea: Ostracoda) de pozas temporales" presentada por JOSEP ANTONI AGUILAR ALBEROLA para optar al grado de Doctor en Ciencias Biológicas por la Universitat de València. Firmado: Josep Antoni Aguilar Alberola Tesis dirigida por el Doctor en Ciencias Biológicas por la Universitat de València, FRANCESC MESQUITA JOANES. Firmado: F. Mesquita i Joanes Profesor Titular de Ecología Universitat de València A Laura, Paco, i la meua família Resumen Los ostrácodos son un grupo de pequeños crustáceos con amplia distribución mundial, cuyo cuerpo está protegido por dos valvas laterales que suelen preservarse con facilidad en el sedimento. En el presente trabajo se muestra la primera cita del ostrácodo Heterocypris bosniaca Petkowski, Scharf y Keyser, 2000 para la Península Ibérica. Se trata de una especie de cipridoideo muy poco conocida que habita pozas de aguas temporales. Se descubrió el año 2000 en Bosnia y desde entonces solo se ha reportado su presencia en Israel (2004) y en Valencia (presente trabajo). -

Fossil Calibrations for the Arthropod Tree of Life
bioRxiv preprint doi: https://doi.org/10.1101/044859; this version posted June 10, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY 4.0 International license. FOSSIL CALIBRATIONS FOR THE ARTHROPOD TREE OF LIFE AUTHORS Joanna M. Wolfe1*, Allison C. Daley2,3, David A. Legg3, Gregory D. Edgecombe4 1 Department of Earth, Atmospheric & Planetary Sciences, Massachusetts Institute of Technology, Cambridge, MA 02139, USA 2 Department of Zoology, University of Oxford, South Parks Road, Oxford OX1 3PS, UK 3 Oxford University Museum of Natural History, Parks Road, Oxford OX1 3PZ, UK 4 Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK *Corresponding author: [email protected] ABSTRACT Fossil age data and molecular sequences are increasingly combined to establish a timescale for the Tree of Life. Arthropods, as the most species-rich and morphologically disparate animal phylum, have received substantial attention, particularly with regard to questions such as the timing of habitat shifts (e.g. terrestrialisation), genome evolution (e.g. gene family duplication and functional evolution), origins of novel characters and behaviours (e.g. wings and flight, venom, silk), biogeography, rate of diversification (e.g. Cambrian explosion, insect coevolution with angiosperms, evolution of crab body plans), and the evolution of arthropod microbiomes. We present herein a series of rigorously vetted calibration fossils for arthropod evolutionary history, taking into account recently published guidelines for best practice in fossil calibration. -

Soft Body Morphology, Dissection and Slide-Preparation of Ostracoda: a Primer
Joannea Geol. Paläont. 11: 327-343 (2011) Soft body morphology, dissection and slide-preparation of Ostracoda: a primer Tadeusz NAMIOTKO, Dan L. DANIELOPOL & Angel BALTANÁS Abstract: Most commonly used techniques for treating ostracod soft body for taxonomi- cal purposes with optical microscopy are described with emphasis on the order Podo- copida. A variety of procedures for pre-treatment, storage, recovery of dried specimens, dissection, temporary and permanent mounting, and staining methods are presented and evaluated. General morphology and terminology of the ostracod appendages are also summarised. Key Words: Ostracoda; Dissection; Mounting; Staining; Appendages; Morphology. 1. Introduction One of best diagnostic and most conspicuous trait of Ostracoda is a bivalved carapace that may completely envelop the whole animal body with limbs. Ostracodologists usu- ally refer to calcified valves as “hard parts”, whereas to the appendages and other in- ternal organs as „soft parts”. The classification of Recent ostracods is based mainly on differences in the soft part morphology which is ordinarily of little use by palaeontolo- gists. However, considering the close functional relationship often existing between the soft and hard parts, each expressing to a considerable extent the morphology of the other, even basic knowledge on the appendages without doubt should help palaeonto- logists to better understand and interpret various features displayed in the valves of the fossil ostracods. Examining living material collected from modern habitats enables pa- laeontologists also to become familiar with intraspecific variability or ecology, which could greatly facilitate both taxonomical and palaeoenvironmental studies. Therefore the morphology and terminology of the soft parts of ostracods is outlined (focusing on limbs) in this primer prior to the description of various laboratory techniques involved in the appendage preparation for optical microscopy. -

Sepkoski, J.J. 1992. Compendium of Fossil Marine Animal Families
MILWAUKEE PUBLIC MUSEUM Contributions . In BIOLOGY and GEOLOGY Number 83 March 1,1992 A Compendium of Fossil Marine Animal Families 2nd edition J. John Sepkoski, Jr. MILWAUKEE PUBLIC MUSEUM Contributions . In BIOLOGY and GEOLOGY Number 83 March 1,1992 A Compendium of Fossil Marine Animal Families 2nd edition J. John Sepkoski, Jr. Department of the Geophysical Sciences University of Chicago Chicago, Illinois 60637 Milwaukee Public Museum Contributions in Biology and Geology Rodney Watkins, Editor (Reviewer for this paper was P.M. Sheehan) This publication is priced at $25.00 and may be obtained by writing to the Museum Gift Shop, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, WI 53233. Orders must also include $3.00 for shipping and handling ($4.00 for foreign destinations) and must be accompanied by money order or check drawn on U.S. bank. Money orders or checks should be made payable to the Milwaukee Public Museum. Wisconsin residents please add 5% sales tax. In addition, a diskette in ASCII format (DOS) containing the data in this publication is priced at $25.00. Diskettes should be ordered from the Geology Section, Milwaukee Public Museum, 800 West Wells Street, Milwaukee, WI 53233. Specify 3Y. inch or 5Y. inch diskette size when ordering. Checks or money orders for diskettes should be made payable to "GeologySection, Milwaukee Public Museum," and fees for shipping and handling included as stated above. Profits support the research effort of the GeologySection. ISBN 0-89326-168-8 ©1992Milwaukee Public Museum Sponsored by Milwaukee County Contents Abstract ....... 1 Introduction.. ... 2 Stratigraphic codes. 8 The Compendium 14 Actinopoda.