Possible Fossil Ostracod (Crustacea) Eggs from the Cretaceous of Brazil
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ostracoda an Introduction.Pdf
The Ostracoda (from Wikipedia, 5/5/2009: http://en.wikipedia.org/wiki/Ostracod) Ostracoda is a class of the Crustacea, sometimes known as the seed shrimp because of their appearance. Ostracods are small crustaceans, typically around one mm in size, but varying between 0.2 to 30 mm, laterally compressed and protected by a bivalve-like, chitinous or calcareous valve or "shell". The hinge of the two valves is in the upper, dorsal region of the body. Some 65,000 species (13,000 of which are extant taxa) have been identified, grouped into several orders. This group may not be monophyletic. Ostracod taxa are grouped into a Class based on gross morphology. Ecologically, marine ostracods can be part of the zooplankton or (most commonly) they are part of the benthos, living on or inside the upper layer of the sea floor. Many ostracods, especially the Podocopida, are also found in fresh water and some are known from humid continental forest soils. The body consists of a cephalon (head), separated from the thorax by a slight constriction. The segmentation is unclear. The abdomen is regressed or absent whereas the adult gonads are relatively large. There are 5–8 pairs of appendages. The branchial plates are responsible for oxygenation. The epidermal cells may also secrete calcium carbonate after the chitinous layer is formed, resulting in a chalk layer enveloped by chitin. This calcification is not equally pronounced in all orders. During every instar transition, the old carapace (chitinous and calcified) is rejected and a new, larger is formed and calcified. The outer lamella calcifies completely, while the inner lamella calcifies partially, with the rest remaining chitinous. -

Volume 2, Chapter 10-2: Arthropods: Crustacea
Glime, J. M. 2017. Arthropods: Crustacea – Ostracoda and Amphipoda. Chapt. 10-2. In: Glime, J. M. Bryophyte Ecology. Volume 2. 10-2-1 Bryological Interaction. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. Last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 10-2 ARTHROPODS: CRUSTACEA – OSTRACODA AND AMPHPODA TABLE OF CONTENTS CLASS OSTRACODA ..................................................................................................................................... 10-2-2 Adaptations ................................................................................................................................................ 10-2-3 Swimming to Crawling ....................................................................................................................... 10-2-3 Reproduction ....................................................................................................................................... 10-2-3 Habitats ...................................................................................................................................................... 10-2-3 Terrestrial ............................................................................................................................................ 10-2-3 Peat Bogs ............................................................................................................................................ 10-2-4 Aquatic ............................................................................................................................................... -
24 Guide to Crustacea
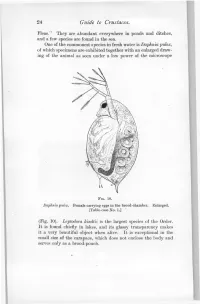
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

15 International Symposium on Ostracoda
Berliner paläobiologische Abhandlungen 1-160 6 Berlin 2005 15th International Symposium on Ostracoda In Memory of Friedrich-Franz Helmdach (1935-1994) Freie Universität Berlin September 12-15, 2005 Abstract Volume (edited by Rolf Kohring and Benjamin Sames) 2 ---------------------------------------------------------------------------------------------------------------------------------------- Preface The 15th International Symposium on Ostracoda takes place in Berlin in September 2005, hosted by the Institute of Geological Sciences of the Freie Universität Berlin. This is the second time that the International Symposium on Ostracoda has been held in Germany, following the 5th International Symposium in Hamburg in 1974. The relative importance of Ostracodology - the science that studies Ostracoda - in Germany is further highlighted by well-known names such as G.W. Müller, Klie, Triebel and Helmdach, and others who stand for the long tradition of research on Ostracoda in Germany. During our symposium in Berlin more than 150 participants from 36 countries will meet to discuss all aspects of living and fossil Ostracoda. We hope that the scientific communities working on the biology and palaeontology of Ostracoda will benefit from interesting talks and inspiring discussions - in accordance with the symposium's theme: Ostracodology - linking bio- and geosciences We wish every participant a successful symposium and a pleasant stay in Berlin Berlin, July 27th 2005 Michael Schudack and Steffen Mischke CONTENT Schudack, M. and Mischke, S.: Preface -

Ecologia D'ostracodes
Ecologia d’ostracodes simbionts (Entocytheridae) de carrancs invasors a Europa Tesi doctoral Alexandre Mestre Pérez Ecologia d’ostracodes simbionts (Entocytheridae) de carrancs invasors a Europa Ecology of symbiotic ostracods (Entocytheridae) inhabiting invasive crayfish in Europe Tesi doctoral 2014 Alexandre Mestre Pérez Departament de Microbiologia i Ecologia Universitat de València Programa de Doctorat en Biodiversitat i Biologia Evolutiva 2014 Ecologia d’ostracodes simbionts (Entocytheridae) de carrancs invasors a Europa Doctorand: Alexandre Mestre Pérez Directors: Francesc Mesquita Joanes Juan Salvador Monrós González La imatge de la portada està composada a partir de la foto d’un carranc de riu americà Pacifas- tacus leniusculus i una foto al microscopi electrònic (feta per Burkhard Scharf) d’una còpula d’ostracodes entocitèrids pertanyents a l’espècie Uncinocythere occidentalis, la qual s’ha tro- bat associada a poblacions exòtiques europees de P. leniusculus en aquest treball. Tesi presentada per Alexandre Mestre Pérez per optar al grau de Doctor en Biologia per la Universitat de València. Firmat: Alexandre Mestre Pérez Tesi dirigida pels doctors Francesc Mesquita Joanes Juan Salvador Monrós González Professors titulars d’Ecologia Universitat de València Firmat: Francesc Mesquita Joanes Firmat: Juan S. Monrós González Aquest treball ha estat finançat per un projecte del Ministeri de Ciència i In- novació (ECOINVADER, CGL2008-01296/BOS) i una beca predoctoral ("Cinc Segles") de la Universitat de València. A ma mare, a mon pare i al meu germà Agraïments Em considere molt afortunat i estic molt agraït d’haver gaudit, durant el llarg camí d’aprenentatge que representa la tesi, d’unes condicions excel lents per poder · desenvolupar aquest treball. -

Crustacea: Ostracoda) in Three Temporary Ponds
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by RERO DOC Digital Library Hydrobiologia (2009) 636:219–232 DOI 10.1007/s10750-009-9952-0 PRIMARY RESEARCH PAPER Dynamics of sexual and parthenogenetic populations of Eucypris virens (Crustacea: Ostracoda) in three temporary ponds Maria Joa˜o Fernandes Martins • Jochen Vandekerkhove • Francesc Mezquita • Olivier Schmit • Juan Rueda • Giampaolo Rossetti • Tadeusz Namiotko Received: 20 May 2009 / Revised: 13 September 2009 / Accepted: 15 September 2009 / Published online: 19 October 2009 Ó Springer Science+Business Media B.V. 2009 Abstract Eucypris virens is a freshwater ostracod This renders the species a potentially valuable in which both sexual reproduction and partheno- model organism to study the ‘queen of evolutionary genesis occur. Sympatric coexistence of both problems’, i.e. why sex is so successful despite its reproductive modes is known in zones of overlap. costs (paradox of sex). In order to maximally exploit this potential, a broad knowledge of the species’ ecology is essential, including an under- standing of its life history and population dynam- ics. Here, the phenology of the species was Electronic supplementary material The online version of followed in three temporary ponds through monthly this article (doi:10.1007/s10750-009-9952-0) contains (Spain) or fortnightly (Poland) samplings, through- supplementary material, which is available to authorized users. out an inundation period. This study confirms the wide ecological tolerances of E. virens. Although Handling editor: K. Martens the species is generally assumed to be univoltine, M. J. F. Martins (&) Á J. Vandekerkhove Á T. Namiotko two hatching periods were observed in the Spanish Laboratory of Limnozoology, Department of Genetics, sites. -

Crustacea, Malacostraca)*
SCI. MAR., 63 (Supl. 1): 261-274 SCIENTIA MARINA 1999 MAGELLAN-ANTARCTIC: ECOSYSTEMS THAT DRIFTED APART. W.E. ARNTZ and C. RÍOS (eds.) On the origin and evolution of Antarctic Peracarida (Crustacea, Malacostraca)* ANGELIKA BRANDT Zoological Institute and Zoological Museum, Martin-Luther-King-Platz 3, D-20146 Hamburg, Germany Dedicated to Jürgen Sieg, who silently died in 1996. He inspired this research with his important account of the zoogeography of the Antarctic Tanaidacea. SUMMARY: The early separation of Gondwana and the subsequent isolation of Antarctica caused a long evolutionary his- tory of its fauna. Both, long environmental stability over millions of years and habitat heterogeneity, due to an abundance of sessile suspension feeders on the continental shelf, favoured evolutionary processes of “preadapted“ taxa, like for exam- ple the Peracarida. This taxon performs brood protection and this might be one of the most important reasons why it is very successful (i.e. abundant and diverse) in most terrestrial and aquatic environments, with some species even occupying deserts. The extinction of many decapod crustaceans in the Cenozoic might have allowed the Peracarida to find and use free ecological niches. Therefore the palaeogeographic, palaeoclimatologic, and palaeo-hydrographic changes since the Palaeocene (at least since about 60 Ma ago) and the evolutionary success of some peracarid taxa (e.g. Amphipoda, Isopo- da) led to the evolution of many endemic species in the Antarctic. Based on a phylogenetic analysis of the Antarctic Tanaidacea, Sieg (1988) demonstrated that the tanaid fauna of the Antarctic is mainly represented by phylogenetically younger taxa, and data from other crustacean taxa led Sieg (1988) to conclude that the recent Antarctic crustacean fauna must be comparatively young. -

Taxonomy of Quaternary Deep-Sea Ostracods from the Western North Atlantic Ocean
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2009 Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean Moriaki Yasuhara National Museum of Natural History, Smithsonian Institution, [email protected] Hisayo Okahashi National Museum of Natural History, Smithsonian Institution, [email protected] Thomas M. Cronin U.S. Geological Survey, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Earth Sciences Commons Yasuhara, Moriaki; Okahashi, Hisayo; and Cronin, Thomas M., "Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean" (2009). USGS Staff -- Published Research. 242. https://digitalcommons.unl.edu/usgsstaffpub/242 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. [Palaeontology, Vol. 52, Part 4, 2009, pp. 879–931] TAXONOMY OF QUATERNARY DEEP-SEA OSTRACODS FROM THE WESTERN NORTH ATLANTIC OCEAN by MORIAKI YASUHARA*, HISAYO OKAHASHI* and THOMAS M. CRONIN *Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, MRC 121, PO Box 37012, Washington, DC 20013-7012, USA; e-mails: [email protected] or [email protected] (M.Y.), [email protected] (H.O.) U.S. Geological Survey, -

Cypris 2016-2017
CYPRIS 2016-2017 Illustrations courtesy of David Siveter For the upper image of the Silurian pentastomid crustacean Invavita piratica on the ostracod Nymphateline gravida Siveter et al., 2007. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, and M.D. Sutton. 2015. A 425-million-year- old Silurian pentastomid parasitic on ostracods. Current Biology 23: 1-6. For the lower image of the Silurian ostracod Pauline avibella Siveter et al., 2012. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, M.D. Sutton, and S.C. Joomun. 2013. A Silurian myodocope with preserved soft-parts: cautioning the interpretation of the shell-based ostracod record. Proceedings of the Royal Society London B, 280 20122664. DOI:10.1098/rspb.2012.2664 (published online 12 December 2012). Watermark courtesy of Carin Shinn. Table of Contents List of Correspondents Research Activities Algeria Argentina Australia Austria Belgium Brazil China Czech Republic Estonia France Germany Iceland Israel Italy Japan Luxembourg New Zealand Romania Russia Serbia Singapore Slovakia Slovenia Spain Switzerland Thailand Tunisia United Kingdom United States Meetings Requests Special Publications Research Notes Photographs and Drawings Techniques and Methods Awards New Taxa Funding Opportunities Obituaries Horst Blumenstengel Richard Forester Franz Goerlich Roger Kaesler Eugen Kempf Louis Kornicker Henri Oertli Iraja Damiani Pinto Evgenii Schornikov Michael Schudack Ian Slipper Robin Whatley Papers and Abstracts (2015-2007) 2016 2017 In press Addresses Figure courtesy of Francesco Versino, -

Journal of Cave and Karst Studies
June 2019 Volume 81, Number 2 JOURNAL OF ISSN 1090-6924 A Publication of the National CAVE AND KARST Speleological Society STUDIES DEDICATED TO THE ADVANCEMENT OF SCIENCE, EDUCATION, EXPLORATION, AND CONSERVATION Published By BOARD OF EDITORS The National Speleological Society Anthropology George Crothers http://caves.org/pub/journal University of Kentucky Lexington, KY Office [email protected] 6001 Pulaski Pike NW Huntsville, AL 35810 USA Conservation-Life Sciences Julian J. Lewis & Salisa L. Lewis Tel:256-852-1300 Lewis & Associates, LLC. [email protected] Borden, IN [email protected] Editor-in-Chief Earth Sciences Benjamin Schwartz Malcolm S. Field Texas State University National Center of Environmental San Marcos, TX Assessment (8623P) [email protected] Office of Research and Development U.S. Environmental Protection Agency Leslie A. North 1200 Pennsylvania Avenue NW Western Kentucky University Bowling Green, KY Washington, DC 20460-0001 [email protected] 703-347-8601 Voice 703-347-8692 Fax [email protected] Mario Parise University Aldo Moro Production Editor Bari, Italy [email protected] Scott A. Engel Knoxville, TN Carol Wicks 225-281-3914 Louisiana State University [email protected] Baton Rouge, LA [email protected] Journal Copy Editor Exploration Linda Starr Paul Burger Albuquerque, NM National Park Service Eagle River, Alaska [email protected] Microbiology Kathleen H. Lavoie State University of New York Plattsburgh, NY [email protected] Paleontology Greg McDonald National Park Service Fort Collins, CO The Journal of Cave and Karst Studies , ISSN 1090-6924, CPM [email protected] Number #40065056, is a multi-disciplinary, refereed journal pub- lished four times a year by the National Speleological Society. -

The Taxonomy and Biogeography of Macrofaunal Ostracod Crustaceans
The taxonomy and biogeography of macrofaunal ostracod crustaceans, with focus on the abyssal benthic Pacific fauna relevant to the CCFZ Ivana Karanovic Hanyang University, Department of Life Science, College of Natural Sciences, Seoul 133-791, Korea University of Tasmania, IMAS, Hobart, TAS, 7001, Australia e-mail: [email protected] Few words about myself Italy(Salerno, 2 years) Serbia (Novi Sad, born) Australia (Perth & Hobart, 10 years) Germany (Hamburg, 2 years) South Korea (Seoul, 3.5 years) • Started working on ostracods 15 years ago • Worked on faunas from all continents (including Antarctica) • and from all environments: from freshwater puddles to deep sea • I don’t particularly like ostracods • I like the fact that ostracods give insight into many aspects of biology General information on ostracods • Named in 1802 by Latreille • Name comes from the Greek óstrakon, meaning shell or tile • Common name in English: “mussel shrimp” or “seed shrimp” • In German it is “Muschelkrebse” • Live in all aquatic habitats on the planet Fossil record Systematics • Previously in the class Maxillopoda • Currently recognized as one of the 7 classes of the phylum Crustacea Currently divided into two subclasses 1. Myodocopa 2. Podocopa Systematics cont. 4a. 1. Subclass Myodocopa 1. Order Myodocopina 2. Order Halocyprida 4b. a) Suborder Halocypridina b) Suborder Cladocopina 2a. Subclass Podocopa 3. Order Platycopida 4. Order Podocopida 4c. a) Suborder Bairdiocopina b) Suborder Cytherocopina 2b. c) Suborder Darwinulocopina d) Suborder Cypridocopina 4d. e) Suborder Sigilliocopina 3. Photo credits: 4e. 1, 2: S.N. Brandao 3: Brandao & Yasuhara 4b, c: D. Keyser 4e: From Maddocks (1972) Morphology a. -

Two New Xylophile Cytheroid Ostracods (Crustacea) from Kuril
Arthropod Systematics & Phylogeny 79, 2021, 171–188 | DOI 10.3897/asp.79.e62282 171 Two new xylophile cytheroid ostracods (Crustacea) from Kuril-Kamchatka Trench, with remarks on the systematics and phylogeny of the family Keysercytheridae, Limno cy- theridae, and Paradoxostomatidae Hayato Tanaka1, Hyunsu Yoo2, Huyen Thi Minh Pham3, Ivana Karanovic3,4 1 Tokyo Sea Life Park, 6-2-3 Rinkai-cho, Edogawa-ku, Tokyo 134-8587, Japan 2 Marine Environmental Research and Information Laboratory (MERIL), 17, Gosan-ro, 148 beon-gil, Gun-po-si, Gyoenggi-do, 15180, South Korea 3 Department of Life Science, Research Institute for Convergence of Basic Science, Hanyang University, Seoul 04763, South Korea 4 Institute for Marine and Antarctic Studies, University of Tasmania, Hobart, Tasmania, Australia http://zoobank.org/E29CD94D-AF08-45D2-A319-674F8282D7F2 Corresponding author: Hayato Tanaka ([email protected]) Received 20 December 2020 Accepted 11 May 2021 Academic Editors Anna Hundsdörfer, Martin Schwentner Published 9 June 2021 Citation: Tanaka H, Yoo H, Pham HTM, Karanovic I (2021) Two new xylophile cytheroid ostracods (Crustacea) from Kuril-Kamchatka Trench, with remarks on the systematics and phylogeny of the family Keysercytheridae, Limnocytheridae, and Paradoxostomatidae. Arthropod Systematics & Phylogeny 79: 171–188. https://doi.org/10.3897/asp.79.e62282 Abstract Keysercythere reticulata sp. nov. and Redekea abyssalis sp. nov., collected from the wood fall submerged in the Kuril-Kamchatka Trench (Northwestern Pacific), are only the second records of the naturally occurring, wood-associated ostracod fauna from a depth of over 5000 m. At the same time, K. reticulata is the second and R. abyssalis is the third representative of their respective genera.