Ostracod Soft-Part Morphology, Dissection and Slide-Preparation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anchialine Cave Biology in the Era of Speleogenomics Jorge L
International Journal of Speleology 45 (2) 149-170 Tampa, FL (USA) May 2016 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Life in the Underworld: Anchialine cave biology in the era of speleogenomics Jorge L. Pérez-Moreno1*, Thomas M. Iliffe2, and Heather D. Bracken-Grissom1 1Department of Biological Sciences, Florida International University, Biscayne Bay Campus, North Miami FL 33181, USA 2Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA Abstract: Anchialine caves contain haline bodies of water with underground connections to the ocean and limited exposure to open air. Despite being found on islands and peninsular coastlines around the world, the isolation of anchialine systems has facilitated the evolution of high levels of endemism among their inhabitants. The unique characteristics of anchialine caves and of their predominantly crustacean biodiversity nominate them as particularly interesting study subjects for evolutionary biology. However, there is presently a distinct scarcity of modern molecular methods being employed in the study of anchialine cave ecosystems. The use of current and emerging molecular techniques, e.g., next-generation sequencing (NGS), bestows an exceptional opportunity to answer a variety of long-standing questions pertaining to the realms of speciation, biogeography, population genetics, and evolution, as well as the emergence of extraordinary morphological and physiological adaptations to these unique environments. The integration of NGS methodologies with traditional taxonomic and ecological methods will help elucidate the unique characteristics and evolutionary history of anchialine cave fauna, and thus the significance of their conservation in face of current and future anthropogenic threats. -

Ostracoda an Introduction.Pdf
The Ostracoda (from Wikipedia, 5/5/2009: http://en.wikipedia.org/wiki/Ostracod) Ostracoda is a class of the Crustacea, sometimes known as the seed shrimp because of their appearance. Ostracods are small crustaceans, typically around one mm in size, but varying between 0.2 to 30 mm, laterally compressed and protected by a bivalve-like, chitinous or calcareous valve or "shell". The hinge of the two valves is in the upper, dorsal region of the body. Some 65,000 species (13,000 of which are extant taxa) have been identified, grouped into several orders. This group may not be monophyletic. Ostracod taxa are grouped into a Class based on gross morphology. Ecologically, marine ostracods can be part of the zooplankton or (most commonly) they are part of the benthos, living on or inside the upper layer of the sea floor. Many ostracods, especially the Podocopida, are also found in fresh water and some are known from humid continental forest soils. The body consists of a cephalon (head), separated from the thorax by a slight constriction. The segmentation is unclear. The abdomen is regressed or absent whereas the adult gonads are relatively large. There are 5–8 pairs of appendages. The branchial plates are responsible for oxygenation. The epidermal cells may also secrete calcium carbonate after the chitinous layer is formed, resulting in a chalk layer enveloped by chitin. This calcification is not equally pronounced in all orders. During every instar transition, the old carapace (chitinous and calcified) is rejected and a new, larger is formed and calcified. The outer lamella calcifies completely, while the inner lamella calcifies partially, with the rest remaining chitinous. -

Proceedings of the 3Rd GBIF Science Symposium Brussels, 18-19 April 2005
Proceedings of the 3rd GBIF Science Symposium Brussels, 18-19 April 2005 Tropical Biodiversity: Science, Data, Conservation Edited by H. Segers, P. Desmet & E. Baus Proceedings of the 3rd GBIF Science Symposium Brussels, 18-19 April 2005 Tropical Biodiversity: Science, Data, Conservation Edited by H. Segers, P. Desmet & E. Baus Recommended form of citation Segers, H., P. Desmet & E. Baus, 2006. ‘Tropical Biodiversity: Science, Data, Conservation’. Proceedings of the 3rd GBIF Science Symposium, Brussels, 18-19 April 2005. Organisation - Belgian Biodiversity Platform - Belgian Science Policy In cooperation with: - Belgian Clearing House Mechanism of the CBD - Royal Belgian Institute of Natural Sciences - Global Biodiversity Information Facility Conference sponsors - Belgian Science Policy 1 Table of contents Research, collections and capacity building on tropical biological diversity at the Royal Belgian Institute of Natural Sciences .........................................................................................5 Van Goethem, J.L. Research, Collection Management, Training and Information Dissemination on Biodiversity at the Royal Museum for Central Africa .......................................................................................26 Gryseels, G. The collections of the National Botanic Garden of Belgium ....................................................30 Rammeloo, J., D. Diagre, D. Aplin & R. Fabri The World Federation for Culture Collections’ role in managing tropical diversity..................44 Smith, D. Conserving -
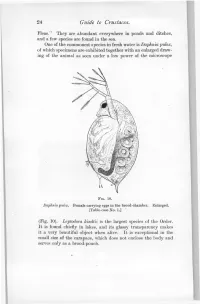
24 Guide to Crustacea
24 Guide to Crustacea. Fleas." They are abundant everywhere in ponds and ditches, and a few species are found in the sea. One of the commonest species in fresh water is Daphnia pulex, of which specimens are exhibited together with an enlarged draw- ing of the animal as seen under a low power of the microscope FIG. 10. Daphnia pulex. Female carrying eggs in the brood-chamber. Enlarged. [Table-case No. 1.] (Fig. 10). Leptodora kindtii is the largest species of the Order. It is found chiefly in lakes, and its glassy transparency makes it a very beautiful object when alive. It is exceptional in the small size of the carapace, which does not enclose the body and serves only as a brood-pouch. Ostracoda. 25 Sub-class II.—OSTRACODA. (Table-ease No. 1.) The number of somites, as indicated by the appendages, is smaller than in any other Crustacea, there being, at most, only two pairs of trunk-limbs behind those belonging to the head- region. The carapace forms a bivalved shell completely en- closing the body and limbs. There is a large, and often leg-like, palp on the mandible. The antennules and antennae are used for creeping or swimming. The Ostracoda (Fig. 10) are for the most part extremely minute animals, and only one or two of the larger species can be exhibited. They occur abundantly in fresh water and in FIG. 11. Shells of Ostracoda, seen from the side. A. Philomedes brenda (Myodocopa) ; B. Cypris fuscata (Podocopa); ('. Cythereis ornata (Podocopa): all much enlarged, n., Notch characteristic of the Myodocopa; e., the median eye ; a., mark of attachment of the muscle connecting the two valves of the shell. -

15 International Symposium on Ostracoda
Berliner paläobiologische Abhandlungen 1-160 6 Berlin 2005 15th International Symposium on Ostracoda In Memory of Friedrich-Franz Helmdach (1935-1994) Freie Universität Berlin September 12-15, 2005 Abstract Volume (edited by Rolf Kohring and Benjamin Sames) 2 ---------------------------------------------------------------------------------------------------------------------------------------- Preface The 15th International Symposium on Ostracoda takes place in Berlin in September 2005, hosted by the Institute of Geological Sciences of the Freie Universität Berlin. This is the second time that the International Symposium on Ostracoda has been held in Germany, following the 5th International Symposium in Hamburg in 1974. The relative importance of Ostracodology - the science that studies Ostracoda - in Germany is further highlighted by well-known names such as G.W. Müller, Klie, Triebel and Helmdach, and others who stand for the long tradition of research on Ostracoda in Germany. During our symposium in Berlin more than 150 participants from 36 countries will meet to discuss all aspects of living and fossil Ostracoda. We hope that the scientific communities working on the biology and palaeontology of Ostracoda will benefit from interesting talks and inspiring discussions - in accordance with the symposium's theme: Ostracodology - linking bio- and geosciences We wish every participant a successful symposium and a pleasant stay in Berlin Berlin, July 27th 2005 Michael Schudack and Steffen Mischke CONTENT Schudack, M. and Mischke, S.: Preface -

2.6 Reproductive System
Edith Cowan University Copyright Warning You may print or download ONE copy of this document for the purpose of your own research or study. The University does not authorise you to copy, communicate or otherwise make available electronically to any other person any copyright material contained on this site. You are reminded of the following: • Copyright owners are entitled to take legal action against persons who infringe their copyright. • A reproduction of material that is protected by copyright may be a copyright infringement. • A court may impose penalties and award damages in relation to offences and infringements relating to copyright material. Higher penalties may apply, and higher damages may be awarded, for offences and infringements involving the conversion of material into digital or electronic form. EDITH COWAN UNIVERSITY EVOLUTION,SYSTEMATICS & GEOGRAPHIC PARTHENOGENESIS OF Ilyodromus (CRUSTACEA,OSTRACODA) by Rylan James Shearn, BSc Hons A thesis submitted to Edith Cowan University in accordance with requirements for the degree of Doctor of Philosophy Submitted 27th February 2015 HAPTER C 2 AN INTRODUCTION TO MORPHOLOGY OFTHE CYPRIDOIDEA (CRUSTACEA,OSTRACODA) 2.1 Abstract Several chapters of this thesis frequently use specialist terminology to iden- tify and describe morphological features of ostracods from the superfam- ily Cypridoidea. To assist the non-specialist reader, this chapter guides the reader through a series of illustrations that serve to explain the fundamen- tal terms and concepts required to interpret these descriptive sections. It is hoped that this chapter will serve as a basic guide, or atlas from which read- ers can refer back to while reading the remainder of the thesis. -

Taxonomy of Quaternary Deep-Sea Ostracods from the Western North Atlantic Ocean
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey 2009 Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean Moriaki Yasuhara National Museum of Natural History, Smithsonian Institution, [email protected] Hisayo Okahashi National Museum of Natural History, Smithsonian Institution, [email protected] Thomas M. Cronin U.S. Geological Survey, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Part of the Earth Sciences Commons Yasuhara, Moriaki; Okahashi, Hisayo; and Cronin, Thomas M., "Taxonomy Of Quaternary Deep-Sea Ostracods From The Western North Atlantic Ocean" (2009). USGS Staff -- Published Research. 242. https://digitalcommons.unl.edu/usgsstaffpub/242 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. [Palaeontology, Vol. 52, Part 4, 2009, pp. 879–931] TAXONOMY OF QUATERNARY DEEP-SEA OSTRACODS FROM THE WESTERN NORTH ATLANTIC OCEAN by MORIAKI YASUHARA*, HISAYO OKAHASHI* and THOMAS M. CRONIN *Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, MRC 121, PO Box 37012, Washington, DC 20013-7012, USA; e-mails: [email protected] or [email protected] (M.Y.), [email protected] (H.O.) U.S. Geological Survey, -

Ostracoda and Foraminifera from Paleocene (Olinda Well), Paraíba Basin, Brazilian Northeast
Anais da Academia Brasileira de Ciências (2017) 89(3): 1443-1463 (Annals of the Brazilian Academy of Sciences) Printed version ISSN 0001-3765 / Online version ISSN 1678-2690 http://dx.doi.org/10.1590/0001-3765201720160768 www.scielo.br/aabc | www.fb.com/aabcjournal Ostracoda and foraminifera from Paleocene (Olinda well), Paraíba Basin, Brazilian Northeast ENELISE K. PIOVESAN¹, ROBBYSON M. MELO¹, FERNANDO M. LOPES², GERSON FAUTH³ and DENIZE S. COSTA³ ¹Laboratório de Geologia Sedimentar e Ambiental/LAGESE, Universidade Federal de Pernambuco, Departamento de Geologia, Centro de Tecnologia e Geociências, Av. Acadêmico Hélio Ramos, s/n, 50740-530 Recife, PE, Brazil ²Instituto Tecnológico de Micropaleontologia/itt Fossil, Universidade do Vale do Rio dos Sinos/UNISINOS, Av. Unisinos, 950, 93022-750 São Leopoldo, RS, Brazil ³PETROBRAS/CENPES/PDEP/BPA, Rua Horácio Macedo, 950, Cidade Universitária, Ilha do Fundão, Prédio 32, 21941-915 Rio de Janeiro, RJ, Brazil Manuscript received on November 7, 2016; accepted for publication on March 16, 2017 ABSTRACT Paleocene ostracods and planktonic foraminifera from the Maria Farinha Formation, Paraíba Basin, are herein presented. Eleven ostracod species were identified in the genera Cytherella Jones, Cytherelloidea Alexander, Eocytheropteron Alexander, Semicytherura Wagner, Paracosta Siddiqui, Buntonia Howe, Soudanella Apostolescu, Leguminocythereis Howe and, probably, Pataviella Liebau. The planktonic foraminifera are represented by the genera Guembelitria Cushman, Parvularugoglobigerina Hofker, Woodringina Loeblich and Tappan, Heterohelix Ehrenberg, Zeauvigerina Finlay, Muricohedbergella Huber and Leckie, and Praemurica Olsson, Hemleben, Berggren and Liu. The ostracods and foraminifera analyzed indicate an inner shelf paleoenvironment for the studied section. Blooms of Guembelitria spp., which indicate either shallow environments or upwelling zones, were also recorded reinforcing previous paleoenvironmental interpretations based on other fossil groups for this basin. -

9<HTOGPC=Ccdhjc>
Life Sciences springer.com/NEWSonline M. A. Alterman, FDA, Bethesda, MD, USA; M. S. Ashton, M. L. Tyrrell, D. Spalding, B. Gentry, C. E. Bullerwell, Mount Allison University, Sackville, P. Hunziker, University of Zurich, Switzerland (Eds) Yale University, New Haven, CT, USA (Eds) NB, Canada (Ed.) Amino Acid Analysis Managing Forest Carbon in a Organelle Genetics Methods and Protocols Changing Climate Evolution of Organelle Genomes and Gene Expression Contents Contents Rapid LC−MS/MS Profiling of Protein Amino Preface.- Acknowledgements.- Chapter 1 Mitochondria and chloroplasts are eukaryotic Acids and Metabolically Related Compounds for Introduction.- Part I: The Science of Forest organelles that evolved from bacterial ancestors Large-Scale Assessment of Metabolic Phenotypes.- Carbon.- Chapter 2 Characterizing organic and harbor their own genomes. The gene products Combination of an AccQ·Tag-Ultra Performance carbon stocks and flows in forest soils.- Chapter of these genomes work in concert with those of Liquid Chromatographic Method with Tandem 3 The physiological ecology of carbon science the nuclear genome to ensure proper organelle Mass Spectrometry for the Analysis of Amino in forest stands.- Chapter 4 Carbon dynamics of metabolism and biogenesis. This book explores the Acids.- Isotope Dilution Liquid Chromatography- tropical forests.- Chapter 5 Carbon dynamics in forces that have shaped the evolution of organelle Tandem Mass Spectrometry for Quantitative temperate forests.- Chapter 6 Carbon dynamics genomes and the expression of -

On a New Species of the Genus Cyprinotus (Crustacea, Ostracoda) from a Temporary Wetland in New Caledonia (Pacific Ocean), With
European Journal of Taxonomy 566: 1–22 ISSN 2118-9773 https://doi.org/10.5852/ejt.2019.566 www.europeanjournaloftaxonomy.eu 2019 · Martens K. et al. This work is licensed under a Creative Commons Attribution License (CC BY 4.0). Research article urn:lsid:zoobank.org:pub:0A180E95-0532-4ED7-9606-D133CF6AD01E On a new species of the genus Cyprinotus (Crustacea, Ostracoda) from a temporary wetland in New Caledonia (Pacifi c Ocean), with a reappraisal of the genus Koen MARTENS 1,*, Mehmet YAVUZATMACA 2 and Janet HIGUTI 3 1 Royal Belgian Institute of Natural Sciences, Freshwater Biology, Vautierstraat 29, B-1000 Brussels, Belgium and University of Ghent, Biology, K.L. Ledeganckstraat 35, B-9000 Ghent, Belgium. 2 Bolu Abant İzzet Baysal University, Faculty of Arts and Science, Department of Biology, 14280 Gölköy Bolu, Turkey. 3 Universidade Estadual de Maringá, Núcleo de Pesquisa em Limnologia, Ictiologia e Aquicultura, Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos, Av. Colombo 5790, CEP 87020-900, Maringá, PR, Brazil. * Corresponding author: [email protected] 2 Email: [email protected] 3 Email: [email protected] 1 urn:lsid:zoobank.org:author:9272757B-A9E5-4C94-B28D-F5EFF32AADC7 2 urn:lsid:zoobank.org:author:36CEC965-2BD7-4427-BACC-2A339F253908 3 urn:lsid:zoobank.org:author:3A5CEE33-280B-4312-BF6B-50287397A6F8 Abstract. The New Caledonia archipelago is known for its high level of endemism in both faunal and fl oral groups. Thus far, only 12 species of non-marine ostracods have been reported. After three expeditions to the main island of the archipelago (Grande Terre), about four times as many species were found, about half of which are probably new. -

Non-Commercial Use Only
J. Limnol., 2017; 76(1): 68-84 ORIGINAL ARTICLE DOI: 10.4081/jlimnol.2016.1480 This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC 4.0). Candona alchichica (Podocopida: Candonidae), a new ostracod species from saline, tropical Lake Alchichica, Mexico Sergio COHUO,1* María del Carmen HERNÁNDEZ,2 Liseth PÉREZ,3 Javier ALCOCER4 1Institut für Geosysteme und Bioindikation, Technische Universität Braunschweig, Langer Kamp 19c D-38106 Braunschweig, Germany; 2Universidad Nacional Autónoma de México, Programa de Posgrado en Ciencias del Mar y Limnología, México; 3Universidad Nacional Autónoma de México, Instituto de Geología. Circuito Institutos. Ciudad Universitaria, Delegación Coyoacán. C.P. 4510, Ciudad de México, México; 4Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Proyecto de Investigación en Limnología Tropical, Av. de los Barrios No. 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, México *Corresponding author: [email protected] ABSTRACT In North America, most species of the Candonidae family belong to the genus Candona. These species are frequently found in freshwater ecosystems and in sediment sequences, which makes them valuable tools for paleoenvironmental reconstructions. Knowledge of Mexican Candona species is limited, however, and scant information exists regarding their taxonomy and ecology. Here we describe Candona alchichica, a new ostracod species we suggest being endemic to Lake Alchichica, central Mexico.only The species belongs to the acuminata group of species, based on the presence of 5+1 setae on the second segment of the mandibular palp. It is closely related to C. patzcuaro, C. tahoensis and C. ohioensis, but differs from those species in that the females have an elongated genital field, wide at the base and narrow at the end. -

Cypris 2016-2017
CYPRIS 2016-2017 Illustrations courtesy of David Siveter For the upper image of the Silurian pentastomid crustacean Invavita piratica on the ostracod Nymphateline gravida Siveter et al., 2007. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, and M.D. Sutton. 2015. A 425-million-year- old Silurian pentastomid parasitic on ostracods. Current Biology 23: 1-6. For the lower image of the Silurian ostracod Pauline avibella Siveter et al., 2012. Siveter, David J., D.E.G. Briggs, Derek J. Siveter, M.D. Sutton, and S.C. Joomun. 2013. A Silurian myodocope with preserved soft-parts: cautioning the interpretation of the shell-based ostracod record. Proceedings of the Royal Society London B, 280 20122664. DOI:10.1098/rspb.2012.2664 (published online 12 December 2012). Watermark courtesy of Carin Shinn. Table of Contents List of Correspondents Research Activities Algeria Argentina Australia Austria Belgium Brazil China Czech Republic Estonia France Germany Iceland Israel Italy Japan Luxembourg New Zealand Romania Russia Serbia Singapore Slovakia Slovenia Spain Switzerland Thailand Tunisia United Kingdom United States Meetings Requests Special Publications Research Notes Photographs and Drawings Techniques and Methods Awards New Taxa Funding Opportunities Obituaries Horst Blumenstengel Richard Forester Franz Goerlich Roger Kaesler Eugen Kempf Louis Kornicker Henri Oertli Iraja Damiani Pinto Evgenii Schornikov Michael Schudack Ian Slipper Robin Whatley Papers and Abstracts (2015-2007) 2016 2017 In press Addresses Figure courtesy of Francesco Versino,