Transposition of the Great Arteries in the Neonate in THIS ISSUE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cardiac Surgery in Early Infancy J
Postgrad Med J: first published as 10.1136/pgmj.48.562.478 on 1 August 1972. Downloaded from Postgraduate Medical Journal (August 1972) 48, 478-485. Cardiac surgery in early infancy J. STARK M.D. Hospital for Sick Children, Great Ormond Street, London, W.C. 1 IMPORTANT advances have been made in the diagnosis and treatment of congenital heart disease (CHD) in early infancy. Accurate diagnosis is now possible in the first days or even hours of life. Operation is also 3530- possible at this early age and very often it provides 0 the only chance for survival. The risk of conservative E .4In15 treatment often exceeds the risk of the operation. O- An aggressive approach to the diagnosis and treat- II II II ment of CHD is dictated by statistics. The estimated z a 10 5 incidence of CHD is 6-8/1000 live births. About 500o 963 99 6 of the children born with CHD die before their first 3 1 1 5 1 16 birthday unless effectively treated. The achievements in the treatment of CHD in infancy are the result of 1963~51964 1965 1966 1967 1968 1969 1970 1971 the combined efforts of paediatricians, cardiologists, FIG. 1. Open-heart surgery under I year of age at the copyright. surgeons, anaesthetists and nurses. Many other Great Ormond Street Thoracic Unit, February 1963 to specialists and technical staff contribute to the September 1971 (115 patients). Open columns, survived; diagnosis and treatment. closed columns, died. The experience of the Thoracic Unit, Hospital for Sick Children at Great Ormond Street, forms the Diagnosis basis of this report (unless otherwise stated). -

BAS Talk Linz
Balloon atrial septostomy Dr Aphrodite Tzifa, MD(Res), FRCPCH! ! Director, Paediatric Cardiology Department, ! Mitera Children's Hospital, Athens, Greece September 10, 2003 ! BAS ! ! 1. The technique of atrial septostomy (BAS) was introduced in 1966 by Rashkind! ! 2. The interventional procedure is performed in neonates that are significantly cyanosed with preductal O2 saturations below 75% after birth or that need an atrial septal defect to offload the LA! “A technique for producing an atrial septal defect without thoracotomy or anesthesia is presented. It can be performed rapidly in any cardiac catheterization laboratory.” (William J. Rashkind, 1966) ! History ! ! “...The initial response to this report varied between admiration and horror but, in either case, the procedure stirred the imagination of the “invasive” cardiologists throughout the entire cardiology world and set the stage for all future intracardiac interventional procedures – the true beginning of pediatric and adult interventional cardiology.” (Charles E. Mullins, 1998) ! HOW to CREATE AN ASD? ! ! 1. Septostomy Balloons! 2. Low profile balloon (Tyshak)! 3. Static balloon (Powerflex, Opta)! 4. Cutting balloons! 5. Blade septostomy catheter! 6. Stenting! ! Transeptal puncture or RF perforation may occasionally be needed Need for creation or enlargement of an ASD: ! WHEN? ! ! 1. TGA: In some units, an atrial septostomy is performed routinely after birth, to ensure mixing of the blood at intracardiac level and discontinue the prostaglandin infusion. However, most would not advocate this due to the potential risk of complications in neonates that are otherwise stable. ! 2. Tricuspid or mitral atresia! 3. PA / IVS! 4. TAPVD with restrictive septum! 5. During hybrid procedures for HLHS syndrome! 6. -

Balloon Atrial Septostomy in Complete Transposition of Great Arteries in Infancy
Br Heart J: first published as 10.1136/hrt.32.1.61 on 1 January 1970. Downloaded from British HeartJournal, I970, 32, X6i. Balloon atrial septostomy in complete transposition of great arteries in infancy A. W. Venables From Royal Children's Hospital, Melbourne, Australia The results of 26 completed balloon atrial septostomies in complete arterial transposition in infancy are described, and complications discussed. Of 7 deaths following this procedure, 3 were clearly or probably related to it or to its failure to produce an adequate septal defect, while 4 were unrelated. Atrial perforations occurred on 4 occasions. Necropsy information regarding the defects produced by the procedure is given. Anatomical features of the fossa ovalis appear to determine the size of the defect created. The effect of the procedure is illustrated by photographs of representative necropsy specimens. Apparently adequate initial defects do not guarantee satisfactory long-term palliation, and 4 of iI infants followed for more than 6 months after effective initial palliation have required surgical procedures to provide more adequate atrial mixing of blood. Despite this, the procedure appears to offer considerable advantage over initial surgical procedures to create atrial defects. The value of palliative procedures in com- Subjects and methods plete arterial transposition is indisputable. From to mid-May I23 cases of trans- ig60 i969, http://heart.bmj.com/ Most commonly, atrial septal defects arz cre- position of the great arteries in infancy were diag- ated in order to increase effective pulmonary nosed in the Cardiac Unit of the Royal Children's flow. Since I966 the balloon catheter tech- Hospital, Melbourne. -

ICD-9-CM Procedures (FY10)
2 PREFACE This sixth edition of the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) is being published by the United States Government in recognition of its responsibility to promulgate this classification throughout the United States for morbidity coding. The International Classification of Diseases, 9th Revision, published by the World Health Organization (WHO) is the foundation of the ICD-9-CM and continues to be the classification employed in cause-of-death coding in the United States. The ICD-9-CM is completely comparable with the ICD-9. The WHO Collaborating Center for Classification of Diseases in North America serves as liaison between the international obligations for comparable classifications and the national health data needs of the United States. The ICD-9-CM is recommended for use in all clinical settings but is required for reporting diagnoses and diseases to all U.S. Public Health Service and the Centers for Medicare & Medicaid Services (formerly the Health Care Financing Administration) programs. Guidance in the use of this classification can be found in the section "Guidance in the Use of ICD-9-CM." ICD-9-CM extensions, interpretations, modifications, addenda, or errata other than those approved by the U.S. Public Health Service and the Centers for Medicare & Medicaid Services are not to be considered official and should not be utilized. Continuous maintenance of the ICD-9- CM is the responsibility of the Federal Government. However, because the ICD-9-CM represents the best in contemporary thinking of clinicians, nosologists, epidemiologists, and statisticians from both public and private sectors, no future modifications will be considered without extensive advice from the appropriate representatives of all major users. -

Images Paediatr Cardiol
W Boehm, M Emmel, and N Sreeram. Balloon Atrial Septostomy: History and Technique. Images Paediatr Cardiol. 2006 Jan-Mar; 8(1): 8–14. in PAEDIATRIC CARDIOLOGY IMAGES Images Paediatr Cardiol. 2006 Jan-Mar; 8(1): 8–14. PMCID: PMC3232558 Balloon Atrial Septostomy: History and Technique W Boehm, M Emmel, and N Sreeram University Hospital of Cologne, Germany. Contact information: N. Sreeram, Department of Paediatric Cardiology, University Hospital of Cologne, Kerpenerstrasse 62, 50937 Cologne, Germany Phone: +49 221 47886301 +49 221 47886301 Fax: +49 221 47886302 ; Email: [email protected] MeSH: Transposition of the great arteries, Heart defects, congenital, Catheter, invervention, Balloon atrial septostomy Copyright : © Images in Paediatric Cardiology This is an open-access article distributed under the terms of the Creative Commons Attribution-Noncommercial-Share Alike 3.0 Unported, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Introduction “A technique for producing an atrial septal defect without thoracotomy or anesthesia is presented. It can be performed rapidly in any cardiac catheterization laboratory.” (William J. Rashkind, 1966)1 “...The initial response to this report varied between admiration and horror but, in either case, the procedure stirred the imagination of the “invasive” cardiologists throughout the entire cardiology world and set the stage for all future intracardiac interventional procedures – the true beginning of pediatric and adult interventional cardiology.” (Charles E. Mullins, 1998)2 The natural history of untreated transposition of the great vessels in the neonate is poor. Complete correction has been possible since 1959 with the atrial switch procedure, first described by Senning.3 Mustard simplified this method, reducing mortality rates to a reasonable level.4 Best results with both operations were achieved in children beyond six months of age. -

Left Atrial Decompression by Percutaneous Cannula Placement While on Extracorporeal Membrane Oxygenation
View metadata, citation and similar papers at core.ac.uk brought to you by CORE Brief Communications provided by Elsevier - Publisher Connector Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation Anthony M. Hlavacek, MD, Andrew M. Atz, MD, Scott M. Bradley, MD, and Varsha M. Bandisode, MD, Charleston, SC enoarterial extracorporeal membrane oxygenation serially dilated with 10F and 16F transseptal sheath dilators. With (ECMO) is often used in pediatric patients with severe transesophageal echocardiography guidance, a 17F cannula was myocardial dysfunction, typically after cardiac sur- advanced over the guidewire into the LA and connected to the gery, or in patients with acute myocarditis. A problem venous ECMO circuit. Chest radiography confirmed resolution of Vfrequently encountered in these patients is left atrial hypertension, his pulmonary edema by the subsequent day. An echocardiogram which may result in pulmonary edema, ventricular distention, and revealed normalization of his LA size and an estimated LA pres- subendocardial ischemia.1,2 Decompression of the left atrium (LA) sure decrease to 18 mm Hg by Doppler measurement of the mitral will reduce diastolic pressures in the left heart, resulting in an regurgitation gradient. He remained on ECMO for 42 days until a improvement in coronary perfusion and lessen pulmonary edema. successful orthotopic heart transplant was performed. We describe the percutaneous placement of a venous cannula in the LA while on ECMO in a child with severe -

Interventional Echocardiography: Opportunities and Challenges in an Emerging Field
Henry Ford Health System Henry Ford Health System Scholarly Commons Cardiology Articles Cardiology/Cardiovascular Research 10-23-2020 Interventional echocardiography: Opportunities and challenges in an emerging field James Lee Tiffany Chen Edward Gill Follow this and additional works at: https://scholarlycommons.henryford.com/cardiology_articles Received: 1 June 2020 | Revised: 10 September 2020 | Accepted: 12 September 2020 DOI: 10.1111/echo.14874 REVIEW Interventional echocardiography: Opportunities and challenges in an emerging field James Lee MD1 | Tiffany Chen MD2 | Edward Gill MD3 1Division of Cardiology, Henry Ford Heart and Vascular Institute, Detroit, MI, USA Abstract 2Division of Cardiovascular Medicine, The growth of transcatheter structural heart disease interventions has created a Perelman School of Medicine, Hospital of subspecialty of interventional imagers who focus on preprocedural planning and the the University of Pennsylvania, Philadelphia, PA, USA periprocedural guidance of these complex cases. In particular interventional imag- 3Division of Cardiology, University of ers who focus on periprocedural guidance have developed a specific expertise in Colorado School of Medicine, Aurora, CO, USA interventional transesophageal echocardiography (iTEE). This nascent field has chal- lenges in training, reimbursement, and occupational hazards which are unique to this Correspondence James Lee, MD, Division of Cardiology, field. This review encompasses the evolution of iTEE, current challenges, and future Henry Ford Heart and Vascular -

NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures
NATIONAL QUALITY FORUM National Voluntary Consensus Standards for Pediatric Cardiac Surgery Measures Measure Number: PCS‐001‐09 Measure Title: Participation in a national database for pediatric and congenital heart surgery Description: Participation in at least one multi‐center, standardized data collection and feedback program that provides benchmarking of the physician’s data relative to national and regional programs and uses process and outcome measures. Participation is defined as submission of all congenital and pediatric operations performed to the database. Numerator Statement: Whether or not there is participation in at least one multi‐center data collection and feedback program. Denominator Statement: N/A Level of Analysis: Group of clinicians, Facility, Integrated delivery system, health plan, community/population Data Source: Electronic Health/Medical Record, Electronic Clinical Database (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Clinical Registry (The Society of Thoracic Surgeons Congenital Heart Surgery Database), Electronic Claims, Paper Medical Record Measure Developer: Society of Thoracic Surgeons Type of Endorsement: Recommended for Time‐Limited Endorsement (Steering Committee Vote, Yes‐9, No‐0, Abstain‐0) Attachments: “STS Attachment: STS Procedure Code Definitions” Meas# / Title/ Steering Committee Evaluation and Recommendation (Owner) PCS-001-09 Recommendation: Time-Limited Endorsement Yes-9; No-0; Abstain-0 Participatio n in a Final Measure Evaluation Ratings: I: Y-9; N-0 S: H-4; M-4; L-1 U: H-9; M-0; L-0 national F: H-7; M-2; L-0 database for Discussion: pediatric I: The Steering Committee agreed that this measure is important to measure and report. and By reporting through a database, it is possible to identify potential quality issues and congenital provide benchmarks. -

STS/EACTS Short List Mapping to European Paediatric Cardiac Code Short List with ICD-9 & ICD-10 Crossmapping
12S02-05.qxd 22/Sep/02 1:23 PM Page 50 Cardiol Young 2002; 12 (Suppl. 2): 50–62 © Greenwich Medical Media Ltd./AEPC ISSN 1047-9511 STS/EACTS Short List mapping to European Paediatric Cardiac Code Short List with ICD-9 & ICD-10 crossmapping [Boxed items ϭ codes where 2 or 3 EPCC items required for single STS/EACTS item] STS/EACTS term EPCC term EPCC ICD-9 ICD-9 ICD-10 ICD-10 code additional additional Appendix IIIA – Noncardiac Abnormalities Short List None Hereditary/non-cardiac abnormality not apparent, 10.23.00 nc nc Asplenia Spleen absent (asplenia), 03.07.03 759.0 Q20.6 Polysplenia Multiple spleens (polysplenia), 03.07.04 759.0 Q20.6 Down Syndrome Trisomy 21 – Down’s syndrome, 14.01.02 758.0 Q90.9 Turner Syndrome 45XO – Turner’s syndrome, 14.01.05 758.6 Q96 DiGeorge DiGeorge sequence, 14.02.06 279.1 D82.1 Williams Beuren syndrome Williams syndrome (infantile hypercalcaemia), 14.02.30 275.4 747.2 Q93.8 Alagille syndrome (intrahepatic biliary Alagille syndrome: arteriohepatic dysplasia, 14.02.66 751.6 Q44.7 duct agenesis) 22q11 deletion 22q11 microdeletion – CATCH 22, 14.01.21 758.3 Q93.8 Other chromosomal/syndromic abnormality No exact equivalent 759.9 Q87.8 Rubella Fetal rubella syndrome, 14.02.32 771.0 P35.0 Marfan syndrome Marfan syndrome, 14.02.17 759.8 Q87.4 Other preoperative noncardiac abnormality No exact equivalent Appendix IIIB – General Preoperative Risk Factor Short List None No pre-procedural risk factors, 10.20.00 nc nc Preoperative mechanical circulatory support Pre-procedural mechanical circulatory support, 10.20.15 -
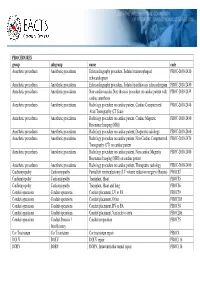
PROCEDURES Group Subgroup Name Code Anesthetic Procedures
PROCEDURES group subgroup name code Anesthetic procedures Anesthetic procedures Echocardiography procedure, Sedated transesophageal PROC-2010-2420 echocardiogram Anesthetic procedures Anesthetic procedures Echocardiography procedure, Sedated transthoracic echocardiogram PROC-2010-2430 Anesthetic procedures Anesthetic procedures Non-cardiovascular, Non-thoracic procedure on cardiac patient with PROC-2010-2435 cardiac anesthesia Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Cardiac Computerized PROC-2010-2440 Axial Tomography (CT Scan) Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Cardiac Magnetic PROC-2010-2450 Resonance Imaging (MRI) Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Diagnostic radiology PROC-2010-2460 Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Non-Cardiac Computerized PROC-2010-2470 Tomography (CT) on cardiac patient Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Non-cardiac Magnetic PROC-2010-2480 Resonance Imaging (MRI) on cardiac patient Anesthetic procedures Anesthetic procedures Radiology procedure on cardiac patient, Therapeutic radiology PROC-2010-2490 Cardiomyopathy Cardiomyopathy Partial left ventriculectomy (LV volume reduction surgery) (Batista) PROC87 Cardiomyopathy Cardiomyopathy Transplant, Heart PROC85 Cardiomyopathy Cardiomyopathy Transplant, Heart and lung PROC86 Conduit operations Conduit operations Conduit placement, -

Balloon Atrial Septostomy Via the Umbilical Vein
Br Heart J: first published as 10.1136/hrt.36.10.1040 on 1 October 1974. Downloaded from Case reports British Heart Journal, I974, 36, I040-IO4. Balloon atrial septostomy via the umbilical vein H. H. Kaye' and Michael Tynan From the Department ofCardiology, Newcastle General Hospital, Westgate Road, Newcastle upon Tyne A case of transposition of the great arteries is presented in which an adequate balloon septostomy was per- formed through the umbilical vein. Some diffxiculty was experienced in traversing the ductus venosus. The possible complications of umbilical vein catheterization are reviewed but it is suggested that some of them may be avoided since the procedure is carried out under x-ray control. The outlook for children with transposition of the left ventricle, but this reverted spontaneously to sinus great arteries has improved in recent years. Balloon rhythm. A diagnosis of transposition of the great atrial septostomy (Rashkind and Miller, I966) has arteries with only an interatrial communication was made. shown to be the Bidirectional shunting was demonstrated before septo- been palliative treatment of choice stomy which could only have been taking place at atrial (Tynan, I97I) and considerable success has been level. An unsuccessful attempt was then made to intro- achieved by surgical correction (Clarkson et al., duce a 5 5 French Rashkind catheter into the right copyright. 1972), particularly in infancy (Breckenridge et al., femoral vein, and owing to extensive damage to the 1972). vein it had to be tied off. Because the baby's condition Technical difficulties, however, can be a problem had deteriorated, it was decided to attempt a balloon in the catheter room and some authors have drawn septostomy via the umbilical vein thus saving time and attention to this when discussing balloon atrial limiting further surgical trauma. -

Overview of Endovascular Atrial Septostomy PDF 164 KB
NATIONAL INSTITUTE FOR CLINICAL EXCELLENCE INTERVENTIONAL PROCEDURES PROGRAMME Interventional procedure overview of balloon or blade atrial septostomy Introduction This overview has been prepared to assist members of IPAC advise on the safety and efficacy of an interventional procedure previously reviewed by SERNIP. It is based on a rapid survey of published literature, review of the procedure by one or more Specialist Advisors and review of the content of the SERNIP file. It should not be regarded as a definitive assessment of the procedure. Date prepared This overview was prepared by Bazian Ltd in March 2003. Procedure name Balloon or blade atrial septostomy including static balloon atrial septostomy Synonym for balloon septostomy: Rashkind septostomy Synonyms for blade septostomy: knife or Park septostomy Specialty society British Paediatric Cardiac Association Indications The main indication for this procedure is transposition of the great arteries; an uncommon congenital cardiac anomaly in which the aorta arises from the right ventricle and the pulmonary trunk arises from the left ventricle. This results in two separate circuits of blood flow, where highly-oxygenated blood keeps cycling through the lungs, while oxygen-depleted blood recycles around the body. As a result, the baby develops a blue colour (cyanosis) shortly after birth. The newborn can survive for a few days because the foramen ovale, a small hole in the foetal interatrial septum, allows some oxygenated blood to mix with the blood that is being circulated around the body. However, the foramen ovale normally closes within a few days after birth. Less commonly, septostomy is carried out in children with other cyanotic congenital abnormalities.