Curcuma Zedoaria) on Growth Performances and Hemato-Biochemical Parameters of Broiler Chicks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Thesis Final Edition
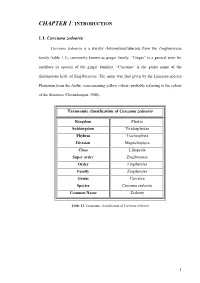
CHAPTER 1: INTRODUCTION 1.1. Curcuma zedoaria Curcuma zedoaria is a starchy rhizomatous/tuberous from the Zingiberaceae family (table 1.1), commonly known as ginger family. “Ginger” is a general term for members or species of the ginger families. “Curcuma” is the genus name of the rhizomatous herb, of Zingiberaceae. The name was first given by the Linnaeus species Plantarum from the Arabic term meaning yellow colour, probably referring to the colour of the rhizomes (Govindarajan, 1980). Taxonomic classification of Curcuma zedoaria Kingdom Plantae Subkingdom Viridaeplantae Phylum Tracheophyta Division Magnoliophyta Class Liliopsida Super order Zingiberanae Order Zingiberales Family Zingiberales Genus Curcuma Curcuma zedoaria Species Common Name Zedoary Table 1.1 Taxonomic classification of Curcuma zedoaria 1 Chapter 1 1.1.1. Description and distribution Curcuma zedoaria is locally known as “kunyit putih” or “temu putih”. It is able to grow up to one and half meters or even more. The leaves are around eighty centimetres long and they usually have a purple-brown flush along the midrib on both surfaces of the leaf. The rhizomes are frequently confused with those of Curcuma aeruginosa because both are of a similar colour (yellow). However, they can be distinguished easily by conducting a cross section on the rhizomes of the mature plants of Curcuma aeruginosa which are slightly dark purplish. In comparison, the colour of the rhizomes of Curcuma zedoaria is pale yellow or white. The rhizomes of Curcuma aeruginosa are highly aromatic due to the high amount of 1, 8-cineol as 25.20% (Ibrahim et al. 2003). Curcuma zedoaria grows mainly in the East-Asian countries including China (called Er- chu in Chinese), Vietnam, India, Bangladesh, Indonesia, Malaysia (can be found at Kuala Selangor, Teluk Intan; Perak, Labis; Johor, and Pahang) and Japan (Islam et al. -

Chemical Composition and Product Quality Control of Turmeric
Stephen F. Austin State University SFA ScholarWorks Faculty Publications Agriculture 2011 Chemical composition and product quality control of turmeric (Curcuma longa L.) Shiyou Li Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Wei Yuan Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Guangrui Deng Ping Wang Stephen F Austin State University, Arthur Temple College of Forestry and Agriculture, [email protected] Peiying Yang See next page for additional authors Follow this and additional works at: http://scholarworks.sfasu.edu/agriculture_facultypubs Part of the Natural Products Chemistry and Pharmacognosy Commons, and the Pharmaceutical Preparations Commons Tell us how this article helped you. Recommended Citation Li, Shiyou; Yuan, Wei; Deng, Guangrui; Wang, Ping; Yang, Peiying; and Aggarwal, Bharat, "Chemical composition and product quality control of turmeric (Curcuma longa L.)" (2011). Faculty Publications. Paper 1. http://scholarworks.sfasu.edu/agriculture_facultypubs/1 This Article is brought to you for free and open access by the Agriculture at SFA ScholarWorks. It has been accepted for inclusion in Faculty Publications by an authorized administrator of SFA ScholarWorks. For more information, please contact [email protected]. Authors Shiyou Li, Wei Yuan, Guangrui Deng, Ping Wang, Peiying Yang, and Bharat Aggarwal This article is available at SFA ScholarWorks: http://scholarworks.sfasu.edu/agriculture_facultypubs/1 28 Pharmaceutical Crops, 2011, 2, 28-54 Open Access Chemical Composition and Product Quality Control of Turmeric (Curcuma longa L.) ,1 1 1 1 2 3 Shiyou Li* , Wei Yuan , Guangrui Deng , Ping Wang , Peiying Yang and Bharat B. Aggarwal 1National Center for Pharmaceutical Crops, Arthur Temple College of Forestry and Agriculture, Stephen F. -

Prioritization of Medicinal Plant for Their Development
PRIORITIZATION OF MEDICINAL PLANT FOR THEIR DEVELOPMENT Criteria for prioritization The National Medicinal Plant Board initially prioritized 32 medicinal plants at national level for their conservation and development. Recently, the list has been revised and 82 species have been included in the list. For the overall development of the medicinal plant sector in the state, there is a need to prioritize various medicinal plant species. This prioritization has to be based on different criteria such as ,(i) criteria for economic development, (ii) Prioritization to address the primary health care of the local community, (iii) medicinal plants prioritized for home and institutional garden, and (iv) prioritization of medicinal plants with conservation value. In the following section we have tried to touch upon different priorities relevant to the state. Medicinal Plants prioritized for trade for high income. The most important criterion they needs to be considered while prioritizing the species for high income is that the plants should be suitable to grow in the prevalent agroclimatic conditions of the state. The species should have high trade value. It should have consistently high demand. The collection, harvest and post harvest technology should suit to the site conditions of Meghalaya.There should have easy access to planting material and it should be comparatively easy to grow. Preference will also be given to those species which are suitable to grow in multi-tier plantations. The selected species should not get easily deteriorated on storage and continued cultivation. They should have enhanced scope for value addition either through primary processing or through secondary processing. A list of top ten prioritized species for obtaining high income through cultivation and trade is given in Table 18. -

Periodic Table of Herbs 'N Spices
Periodic Table of Herbs 'N Spices 11HH 1 H 2 HeHe Element Proton Element Symbol Number Chaste Tree Chile (Vitex agnus-castus) (Capsicum frutescens et al.) Hemptree, Agnus Cayenne pepper, Chili castus, Abraham's balm 118Uuo Red pepper 33LiLi 44 Be 5 B B 66 C 7 N 7N 88O O 99 F 1010 Ne Ne Picture Bear’s Garlic Boldo leaves Ceylon Cinnamon Oregano Lime (Allium ursinum) (Peumus boldus) (Cinnamomum zeylanicum) Nutmeg Origanum vulgare Fenugreek Lemon (Citrus aurantifolia) Ramson, Wild garlic Boldina, Baldina Sri Lanka cinnamon (Myristica fragrans) Oregan, Wild marjoram (Trigonella foenum-graecum) (Citrus limon) 11 Na Na 1212 Mg Mg 1313 Al Al 1414 Si Si 1515 P P 16 S S 1717 Cl Cl 1818 Ar Ar Common Name Scientific Name Nasturtium Alternate name(s) Allspice Sichuan Pepper et al. Grains of Paradise (Tropaeolum majus) (Pimenta dioica) (Zanthoxylum spp.) Perilla (Aframomum melegueta) Common nasturtium, Jamaica pepper, Myrtle Anise pepper, Chinese (Perilla frutescens) Guinea grains, Garden nasturtium, Mugwort pepper, Pimento, pepper, Japanese Beefsteak plant, Chinese Savory Cloves Melegueta pepper, Indian cress, Nasturtium (Artemisia vulgaris) Newspice pepper, et al. Basil, Wild sesame (Satureja hortensis) (Syzygium aromaticum) Alligator pepper 1919 K K 20 Ca Ca 2121 Sc Sc 2222 Ti Ti 23 V V 24 Cr Cr 2525 Mn Mn 2626 Fe Fe 2727 Co Co 2828 Ni Ni 29 Cu Cu 3030 Zn Zn 31 Ga Ga 3232 Ge Ge 3333As As 34 Se Se 3535 Br Br 36 Kr Kr Cassia Paprika Caraway (Cinnamomum cassia) Asafetida Coriander Nigella Cumin Gale Borage Kaffir Lime (Capsicum annuum) (Carum carvi) -

Phytomedicine Ovicidal Effect of Essential Oils from Zingiberaceae
3K\WRPHGLFLQH ² Contents lists available at ScienceDirect Phytomedicine journal homepage: www.elsevier.com/locate/phymed Original Article Ovicidal effect of essential oils from Zingiberaceae plants and Eucalytus 7 globulus on eggs of head lice, Pediculus humanus capitis De Geer Mayura Soonwera⁎, Orawan Wongnet, Sirawut Sittichok Department of Plant Production Technology, Faculty of Agricultural Technology, King Mongkut's Institute of Technology Ladkrabang, Bangkok 10520, Thailand ARTICLE INFO ABSTRACT Keywords: Background: Head lice infestation is an important public health problem worldwide. Chemical pediculicides Ovicidal activity have lost their efficacy because lice have developed resistance to them. Therefore, alternative pediculicides such Head lice egg as essential oils and herbal products have been proposed for treating head lice infestation. Zingiberaceae EOs Study design: To determine the efficacy of essential oils from three Zingiberaceae plants (Curcuma xanthorrhiza, Eucalyptus globulus EO Curcuma zedoaria and Zingiber zerumbet) against head lice eggs and to investigate an augmenting substance (Eucalyptus globulus EO) for improving the efficacy of these essential oils in killing head lice eggs, especially on the inhibition of their hatching process. Permethrin pediculicide, soyabean oil, and drinking water were used as positive, negative, and neutral controls, respectively. Methods: An immersion test was used to evaluate the ovicidal activity of 12 essential oil formulations. Head lice eggs were immersed for 1, 5 and 10 min in the treatments. Mortality rate was observed on day 7 and day 14; mortality was checked under a stereomicroscope. Results: All head lice eggs that were immersed in a combination of 10% C. zedoaria EO and 10% E. globulus EO for 5 min did not hatch at all for 7–14 days of incubation. -

Genus Curcuma
JOURNAL OF CRITICAL REVIEWS ISSN- 2394-5125 VOL 7, ISSUE 16, 2020 A REVIEW ON GOLDEN SPECIES OF ZINGIBERACEAE FAMILY: GENUS CURCUMA Abdul Mubasher Furmuly1, Najiba Azemi 2 1Department of Analytical Chemistry, Faculty of Chemistry, Kabul University, Jamal Mina, 1001 Kabul, Kabul, Afghanistan 2Department of Chemistry, Faculty of Education, Balkh University, 1701 Balkh, Mazar-i-Sharif, Afghanistan Corresponding author: [email protected] First Author: [email protected] Received: 18 March 2020 Revised and Accepted: 20 June 2020 ABSTRACT: The genus Curcuma pertains to the Zingiberaceae family and consists of 70-80 species of perennial rhizomatous herbs. This genus originates in the Indo-Malayan region and it is broadly spread all over the world across tropical and subtropical areas. This study aims to provide more information about morphological features, biological activities, and phytochemicals of genus Curcuma for further advanced research. Because of its use in the medicinal and food industries, Curcuma is an extremely important economic genus. Curcuma species rhizomes are the source of a yellow dye and have traditionally been utilized as spices and food preservers, as a garnishing agent, and also utilized for the handling of various illnesses because of the chemical substances found in them. Furthermore, Because of the discovery of new bioactive substances with a broad range of bioactivities, including antioxidants, antivirals, antimicrobials and anti-inflammatory activities, interest in their medicinal properties has increased. Lack of information concerning morphological, phytochemicals, and biological activities is the biggest problem that the researcher encountered. This review recommended that collecting information concerning the Curcuma genus may be providing more opportunities for further advanced studies lead to avoid wasting time and use this information for further research on bioactive compounds which are beneficial in medicinal purposes KEYWORDS: genus Curcuma; morphology; phytochemicals; pharmacological 1. -

Herbs, Spices and Essential Oils
Printed in Austria V.05-91153—March 2006—300 Herbs, spices and essential oils Post-harvest operations in developing countries UNITED NATIONS INDUSTRIAL DEVELOPMENT ORGANIZATION Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria Telephone: (+43-1) 26026-0, Fax: (+43-1) 26926-69 UNITED NATIONS FOOD AND AGRICULTURE E-mail: [email protected], Internet: http://www.unido.org INDUSTRIAL DEVELOPMENT ORGANIZATION OF THE ORGANIZATION UNITED NATIONS © UNIDO and FAO 2005 — First published 2005 All rights reserved. Reproduction and dissemination of material in this information product for educational or other non-commercial purposes are authorized without any prior written permission from the copyright holders provided the source is fully acknowledged. Reproduction of material in this information product for resale or other commercial purposes is prohibited without written permission of the copyright holders. Applications for such permission should be addressed to: - the Director, Agro-Industries and Sectoral Support Branch, UNIDO, Vienna International Centre, P.O. Box 300, 1400 Vienna, Austria or by e-mail to [email protected] - the Chief, Publishing Management Service, Information Division, FAO, Viale delle Terme di Caracalla, 00100 Rome, Italy or by e-mail to [email protected] The designations employed and the presentation of material in this information product do not imply the expression of any opinion whatsoever on the part of the United Nations Industrial Development Organization or of the Food and Agriculture Organization of the United Nations concerning the legal or development status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

21 CFR Ch. I (4–1–00 Edition)
§ 182.10 21 CFR Ch. I (4±1±00 Edition) generally be regarded as safe for the 186 of this chapter; ``food additive regu- purpose intended, by experts qualified lation'' under parts 170 through 180 of to evaluate its safety. this chapter; ``interim food additive (c) The inclusion of substances in the regulation'' under part 180 of this chap- list of nutrients does not constitute a ter; or ``prohibited from use in food'' finding on the part of the Department under part 189 of this chapter. that the substance is useful as a sup- plement to the diet for humans. [42 FR 14640, Mar. 15, 1977, as amended at 53 (d) Substances that are generally rec- FR 44875, Nov. 7, 1988] ognized as safe for their intended use within the meaning of section 409 of § 182.10 Spices and other natural seasonings and flavorings. the act are listed in this part. When the status of a substance has been re- Spices and other natural seasonings evaluated, it will be deleted from this and flavorings that are generally rec- part, and will be issued as a new regu- ognized as safe for their intended use, lation under the appropriate part, e.g., within the meaning of section 409 of ``affirmed as GRAS'' under part 184 or the Act, are as follows: Common name Botanical name of plant source Alfalfa herb and seed .............................................. Medicago sativa L. Allspice .................................................................... Pimenta officinalis Lindl. Ambrette seed ......................................................... Hibiscus abelmoschus L. Angelica ................................................................... Angelica archangelica L. or other spp. of Angelica. Angelica root ........................................................... Do. Angelica seed .......................................................... Do. Angostura (cusparia bark) ...................................... -

21 CFR Ch. I (4–1–17 Edition) § 182.20
§ 182.20 21 CFR Ch. I (4–1–17 Edition) Common name Botanical name of plant source Marigold, pot ........................................................... Calendula officinalis L. Marjoram, pot .......................................................... Majorana onites (L.) Benth. Marjoram, sweet ...................................................... Majorana hortensis Moench. Mustard, black or brown ......................................... Brassica nigra (L.) Koch. Mustard, brown ....................................................... Brassica juncea (L.) Coss. Mustard, white or yellow ......................................... Brassica hirta Moench. Nutmeg .................................................................... Myristica fragrans Houtt. Oregano (oreganum, Mexican oregano, Mexican Lippia spp. sage, origan). Paprika .................................................................... Capsicum annuum L. Parsley .................................................................... Petroselinum crispum (Mill.) Mansf. Pepper, black .......................................................... Piper nigrum L. Pepper, cayenne ..................................................... Capsicum frutescens L. or Capsicum annuum L. Pepper, red ............................................................. Do. Pepper, white .......................................................... Piper nigrum L. Peppermint .............................................................. Mentha piperita L. Poppy seed ............................................................ -

IJPST Volume 4, Nomor 2 , Juni 2017
IJPST Volume 4, Nomor 2 , Juni 2017 THE EFFECT OF EXTRACTION CONDITION ON THE POLYPHENOL CONTENT AND ANTIOXIDANT ACTIVITY OF Curcuma zedoaria (Christm.) ROSCOE RHIZOME Lia Marliani, Wempi Budiana, dan Yonara Anandari Sekolah Tinggi Farmasi Bandung, Bandung, Indonesia ABSTRAK White turmeric (Curcuma zedoaria (Christm.) Roscoe) is one of Indonesian herbal medicine. The extraction process to get polyphenol compound from natural product was influenced by some factor such as solvent, temperature, time, and method of extraction. The objective of this study was to determine the significant factor of extraction that is solvent, temperature and time of extraction on the polyphenol content and antioxidant activity of white turmeric (Curcuma zedoaria (Christm.) Roscoe) rhizome. Extraction was done by dynamic maceration method with variations (23 factor variable design) of solvent (ethanol 96% and water), temperature (25°C and 70°C), time (6 and 24 hours). The method of analysis of polyphenol content using Folin Ciocalteu reagent, and the antioxidant activity using DPPH free radical reduction method. The experiment design and data analysis using Design-Expert® Software Version 10. The result showed that extraction using ethanol 96% at 70°C for 24 hours was gave high polyphenol content and antioxidant activity. Data analysis was showed that polyphenol content and antioxidant activity was influenced only by solvent of extraction. This study indicated that solvent is significant extraction factor for polyphenol content and antioxidant activity of white turmeric (Curcuma zedoaria (Christm.) Roscoe) rhizome. Keywords: Antioxidant, Curcuma zedoaria (Christm.) Roscoe, extraction, polyphenol Pengaruh Kondisi Ekstraksi terhadap Kandungan Polifenol dan Aktivitas Antioksidan Rimpang Curcuma zedoaria (Christm.) Roscoe ABSTRACT Temu Putih (Curcuma zedoaria (Christm.) Roscoe) adalah salah satu obat herbal Indonesia. -
Show Activity
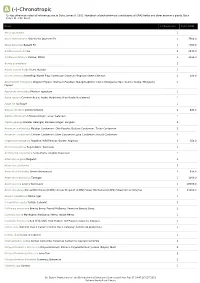
A (-)-Chronotropic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies spectabilis 1 Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Abies balsamea Balsam Fir 1 4090.0 Achillea moschata Iva 2 4536.0 Achillea millefolium Yarrow; Milfoil 3 3190.0 Acinos suaveolens 2 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Sweetflag; Myrtle Flag; Sweetroot; Calamus; Flagroot; Sweet Calamus 2 200.0 Aframomum melegueta Alligator Pepper; Grains-of-Paradise; Malagettapfeffer (Ger.); Malagueta (Sp.); Guinea Grains; Melegueta 1 Pepper Ageratum conyzoides Mexican ageratum 1 Ajuga reptans Common Bugle; Bugle; Bugleherb; Blue Bugle; Bugleweed 1 Ajuga iva Ivy Bugle 1 Aloysia citrodora Lemon Verbena 2 840.0 Alpinia officinarum Chinese Ginger; Lesser Galangal 1 Alpinia galanga Greater Galangal; Siamese Ginger; Languas 3 Amomum xanthioides Malabar Cardamom; Chin Kousha; Bastard Cardamom; Tavoy Cardamom 2 Amomum compactum Chester Cardamom; Siam Cardamom; Java Cardamom; Round Cardamom 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 150.0 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Serpentaria; Virginia Snakeroot 1 Artemisia vulgaris Mugwort 2 Artemisia salsoloides 3 Artemisia herba-alba Desert Wormwood 3 638.0 Artemisia dracunculus Tarragon 1 1000.0 Artemisia cina Levant Wormseed 1 48000.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Sweet Wormwood -

Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line
Medicines 2019, 6, 63; doi:10.3390/medicines6020063 S1 of S6 Supplementary Materials: Screening of Crude Drugs Used in Japanese Kampo Formulas for Autophagy-Mediated Cell Survival of the Human Hepatocellular Carcinoma Cell Line Shinya Okubo, Hisa Komori, Asuka Kuwahara, Tomoe Ohta, Yukihiro Shoyama and Takuhiro Uto Table S1. List of crude drugs. Drug Japanese Name English Name Scientific Name Medicinal Part No. 1 Akyo Donkey Glue Equus asinus glue 2 Ireisen Clematis Root Clematis chinensis, C. mandshurica, C. hexapetala root with rhizome 3 Inchinko Artemisia Capillaris Flower Artemisia capillaris capitulum 4 Uikyo Fennel Foeniculum vulgare fruit 5 Uzu a) Aconite Root Aconitum carmichaeli, A. japonicum tuberous root (mother root) 6 Uyaku Lindera Root Lindera strychnifolia root 7 Engosaku Corydalis Tuber Corydalis turtschaninovii tuber 8 Ogi Astragalus Root Astragalus membranaceus, A. mongholicus root 9 Ogon Scutellaria Root Scutellaria baicalensis root 10 Obaku Phellodendron Bark Phellodendron amurense, P. chinense bark 11 Oren Coptis Rhizome Coptis japonica, C. chinensis, C. deltoidea, C. teeta rhizome 12 Onji Polygala Root Polygala tenuifolia root or root bark 13 Gaiyo Artemisia Leaf Artemisia princeps, A. montana leaf and twig 14 Kashi Myrobalan Fruit Terminalia chebula fruit 15 Kashu Polygonum Root Polygonum multiflorum root 16 Gajutsu Zedoary Curcuma zedoaria rhizome 17 Kakko Pogostemon Herb Pogostemon cablin aerial part 18 Kakkon Pueraria Root Pueraria lobata root 19 Kasseki Aluminum Silicate Hydrate with Silicon Dioxide 20 Karokon Trichosanthes Root Trichosanthes kirilowii, T. kirilowii var. japonica, T. bracteata root Medicines 2019, 6, 63; doi:10.3390/medicines6020063 S2 of S6 21 Karonin Trichosanthes Seed Trichosanthes kirilowii, T. kirilowii var. japonica, T.