Evicore Spine Surgery Guidelines 2018
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spinal Interventional Pain Management and Lumbar Spine Surgery
Spinal Interventional Pain Management and Lumbar Spine Surgery Policy Number: Original Effective Date: MM.06.024 01/01/2014 Line(s) of Business: Current Effective Date: HMO; PPO; QUEST Integration 12/15/2017 Section: Surgery; Medicine Place(s) of Service: Office; Outpatient; Inpatient I. Description The following spinal interventional pain management and lumbar spine surgery procedures require precertification through Magellan Hawaii, formally known as National Imaging Associates, Inc. (NIA): A. Spinal Epidural Injections B. Paravertebral Facet Joint Denervation (radiofrequency neurolysis) C. Paravertebral Facet Joint Injections or Blocks D. Sacroiliac joint injections E. Lumbar Spinal Fusion Surgery II. Administrative Guidelines A. The ordering physician can obtain precertification or consult with Magellan Hawaii by accessing their website at http://www.radmd.com/ or by calling 1 (866) 306-9729, from 6 a.m. to 6 p.m., weekdays, Hawaii Time. Refer to the e-library for instructions on navigating the radmd.com website (RadMD Get Started) and requesting precertification/checking the status of a request (RadMD QuickStart). B. For access to the latest clinical guidelines used for precertification, go to www.radmd.com and click on the link entitled View Clinical Guidelines. C. For interventional pain management procedures (epidural injections, facet joint denervation neurolysis, facet joint injections and sacroiliac joint injections), if more than one procedure is planned, a separate precertification number must be obtained for each procedure planned. D. For spinal surgeries (lumbar fusions, lumbar decompressions, and lumbar microdiscectomy), one precertification number should be obtained for the most invasive surgery to be performed. E. Precertification requirements for injection procedures apply only to office and outpatient services (POS 11, 22, or 24). -

Lumbar Laminectomy Or Laminotomy
Patient Instructions: Lumbar Laminectomy or Laminotomy Surgical Technique A lumbar laminectomy or laminotomy is a surgical approach performed from the back of the lumbar spine. It is usually done through an incision in the middle of the back. Using minimally invasive techniques a small window of bone is drilled in the lamina to allow the surgeon to unpinch the underlying nerves (laminotomy), or in more severe cases the bone is removed completely on both sides to allow nerves on both sides of the spinal canal to be decompressed (laminectomy). It is done using an operating microscope and microsurgical technique. It is used to treat spinal stenosis or lateral recess stenosis and alleviate the pain and/or numbness that occurs in a patients lower back or legs. It can many times be performed on an outpatient basis without the need for an overnight stay in a hospital. Before Surgery • Seven days prior to surgery, please do not take any anti-inflammatory NSAID medications (Celebrex, Ibuprofen, Aleve, Naprosyn, Advil, etc.) as this could prolong your bleeding time during surgery. • Do not eat or drink anything after midnight the day before surgery. This means nothing to drink the morning of surgery except you may take your normal medication with a sip of water if needed. This includes your blood pressure medicine, which in general should be taken. Consult your surgeon or primary care doctor regarding insulin if you take it. • Please do not be late to check in on the day of surgery or it may be cancelled. • Please bring your preoperative folder with you to the surgery and have it when you check in. -
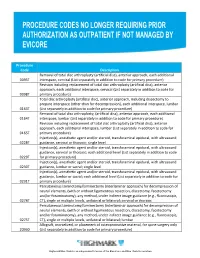
Procedure Codes No Longer Requiring Prior
Procedure Code Description Removal of total disc arthroplasty (artificial disc), anterior approach, each additional 0095T interspace, cervical (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, cervical (List separately in addition to code for 0098T primary procedure) Total disc arthroplasty (artificial disc), anterior approach, including discectomy to prepare interspace (other than for decompression), each additional interspace, lumbar 0163T (List separately in addition to code for primary procedure) Removal of total disc arthroplasty, (artificial disc), anterior approach, each additional 0164T interspace, lumbar (List separately in addition to code for primary procedure) Revision including replacement of total disc arthroplasty (artificial disc), anterior approach, each additional interspace, lumbar (List separately in addition to code for 0165T primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0228T guidance, cervical or thoracic; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound guidance, cervical or thoracic; each additional level (List separately in addition to code 0229T for primary procedure) Injection(s), anesthetic agent and/or steroid, transforaminal epidural, with ultrasound 0230T guidance, lumbar or sacral; single level Injection(s), anesthetic agent and/or steroid, transforaminal epidural, -

Diagnosis and Treatment of Lumbar Disc Herniation with Radiculopathy
Y Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines 1 G Evidence-Based Clinical Guidelines for Multidisciplinary ETHODOLO Spine Care M NE I DEL I U /G ON Diagnosis and Treatment of I NTRODUCT Lumbar Disc I Herniation with Radiculopathy NASS Evidence-Based Clinical Guidelines Committee D. Scott Kreiner, MD Paul Dougherty, II, DC Committee Chair, Natural History Chair Robert Fernand, MD Gary Ghiselli, MD Steven Hwang, MD Amgad S. Hanna, MD Diagnosis/Imaging Chair Tim Lamer, MD Anthony J. Lisi, DC John Easa, MD Daniel J. Mazanec, MD Medical/Interventional Treatment Chair Richard J. Meagher, MD Robert C. Nucci, MD Daniel K .Resnick, MD Rakesh D. Patel, MD Surgical Treatment Chair Jonathan N. Sembrano, MD Anil K. Sharma, MD Jamie Baisden, MD Jeffrey T. Summers, MD Shay Bess, MD Christopher K. Taleghani, MD Charles H. Cho, MD, MBA William L. Tontz, Jr., MD Michael J. DePalma, MD John F. Toton, MD This clinical guideline should not be construed as including all proper methods of care or excluding or other acceptable methods of care reason- ably directed to obtaining the same results. The ultimate judgment regarding any specific procedure or treatment is to be made by the physi- cian and patient in light of all circumstances presented by the patient and the needs and resources particular to the locality or institution. I NTRODUCT 2 Lumbar Disc Herniation with Radiculopathy | NASS Clinical Guidelines I ON Financial Statement This clinical guideline was developed and funded in its entirety by the North American Spine Society (NASS). All participating /G authors have disclosed potential conflicts of interest consistent with NASS’ disclosure policy. -

Commercial Musculoskeletal Codes
Updated January 2018 Commercial Musculoskeletal Codes Investigational or Non-Covered Spine Surgery Pain Management Joint Surgery Codes associated with an Arthrogram CPT Description Commercial Notes Partial excision of posterior vertebral component (eg, spinous 22100 process, lamina or facet) for intrinsic bony lesion, single vertebral segment; cervical 22101 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; thoracic 22102 Partial excision of posterior vertebral component (eg, spinous process, lamina or facet) for intrinsic bony lesion, single vertebral segment; lumbar Partial excision of posterior vertebral component (eg, spinous process, 22103 lamina or facet) for intrinsic bony lesion, single vertebral segment; each additional segment (List separately in addition to code for primary procedure) Partial excision of vertebral body, for intrinsic bony lesion, without 22110 decompression of spinal cord or nerve root(s), single vertebral segment;cervical Partial excision of vertebral body, for intrinsic bony lesion, without 22112 decompression of spinal cord or nerve root(s), single vertebral segment; thoracic Partial excision of vertebral body, for intrinsic bony lesion, without 22114 decompression of spinal cord or nerve root(s), single vertebral segment; lumbar each additional vertebral segment (list separately in addition to code 22116 for primary procedure) Osteotomy of spine, posterior or posterolateral approach, 3 columns, 22206 1 vertebral -

Laminectomy, Facetectomy, Discectomy, Laminotomy, Foraminotomy
www.caryortho.com www.mathurspinesurgery.com Instructions for before and after Surgery Laminectomy, Facetectomy, Discectomy, Laminotomy, Foraminotomy Before You Have Surgery Do not eat or drink after midnight the night before surgery. Please take all routine medications the day of surgery unless otherwise directed by Dr. Mathur, his Nurse (Melissa), or Hospital Staff. Medications to stop prior to surgery: ________________________________ _______________________________________ ________________________________ _______________________________________ You may stay overnight in the hospital. Please bring your brace to the hospital the day of surgery. While you recover at home it is important you protect your spine as it heals. This can best be achieved by following the below instructions and contacting your nurse with any questions or concerns. When You Return Home Your initial activity level will be influenced by the anesthetic agent you have received. It is not uncommon to feel drowsy or tired for a number of hours. Rest when you need it. Symptoms may peak at 2-5 days after surgery, and then will begin to subside. Diet: • Return to your normal diet slowly as tolerated. • Calories and protein are very important for the healing process. Restricting these is not recommended during this time. Back Brace: • Remove your brace to sleep and shower. • You will wear your brace when sitting or standing longer than 10minutes. • You are to wear your back brace until you come to your first post op appointment. At this time we will discuss further use of the brace. It is commonly needed until 6 weeks after surgery. • The purpose of your brace is to stabilize your back to allow for superior healing. -

Lumbar Decompressive Laminectomy Or
nal of S ur pi o n J e Knight et al., J Spine 2013, S2 Journal of Spine DOI: 10.4172/2165-7939.S2-006 ISSN: 2165-7939 Research Article Open Access Lumbar Decompressive Laminectomy or Laminotomy for Degenerative Conditions: “Outcome Comparison of Traditional Open versus Less Invasive Techniques” Reginald Q Knight1,4, Melissa Scribani2, Nicole Krupa2, Scott Grainger1, Craig Goldberg3, Carl Spivak1,4 and Paul Jenkins2 1Bassett Spine Care Institute, Cooperstown, New York, USA 2Bassett Research Institute, Cooperstown, New York, USA 3St. Peters Hospital, Latham, New York, USA 4Columbia University College of Physicians and Surgeons, New York, USA Abstract Study design: Non-randomized chart review of elective lumbar decompression Objective: Compare patient outcome and health system economic impact associated with direct lumbar decompression. Summary of background data: Degenerative lumbar conditions refractory to non-operative measures are traditionally treated via open decompression. Less invasive techniques assisted by tubular retractors or endoscopic visualization continue to grow in popularity. Methods: 338 consecutive patients with spinal stenosis or disc herniation were treated with: Open, Tube-assisted, or Endoscope-assisted procedures based on the surgeons’ typical indications, practice pattern and procedure of choice. Cases stratified by stenosis requiring decompression without discectomy (Stenosis) or disc herniation requiring discectomy (Disc). Data collected preoperatively, one, four and ten months postoperatively. Within strata, perioperative demographics, intraoperative and postoperative complications, and functional outcomes were compared across procedure types. Outcome measures include VAS (back / leg), Oswestry and Medicare subset for Net revenue. Results: 234 Disc and 104 Stenosis cases. Stenosis patients were significantly older than Disc patients (67.0 vs. -

Dynamic Intraspinous Stabilization Reduces Spinal Mobility After
nal of S ur pi o n J e Guentchev et al., J Spine 2017, 6:2 Journal of Spine DOI: 10.4172/2165-7939.1000366 ISSN: 2165-7939 Research Article Open Access Dynamic Intraspinous Stabilization reduces Spinal Mobility After Bilateral Laminotomy Marin Guentchev1,2#, Levente Peter1#, Christian Preuss1, Martin HM Sailer3 and Jochen Tuettenberg1* 1Trinity Medical Center, Spine Unit, 117 Zaichar St, BG-1309 Sofia, Bulgaria 2Department of Neurosurgery, Klinikum Idar- Oberstein, D-55743 Idar-Oberstein, Germany 3Department of Neurosurgery and Spine Surgery, Schulthess Klinik, Lengghalde 2, CH-8008 Zurich, Switzerland #Both have contributed equally to this manuscript Abstract Dynamic stabilization devices were developed to reduce spinal hypermobility while preserving a certain degree of physiological motion. Our goal was to assess radiographic and clinical outcomes of patients treated with surgical decompression and stabilization with the LimiflexTM implant. We investigated the effect of LimiFlexTM implantation on post-operative translation and angulation in 36 patients with spinal stenosis and degenerative spondylolisthesis Meyerding Grade I treated with decompression and dynamic stabilization. Significant improvements following lumbar decompression were observed. The average Oswestry Disability Index (ODI) score fell from 45.9 Pre-operatively to 29.6 at dismissal and 26.5 at first follow up. The average visual analog scale (VAS) score fell from 7 Pre-operatively to 3 at dismissal and 3 at follow up. Pre-operatively the median translation within the operated segment was 2.0 mm. Post-operatively the translation was reduced to 0.7 mm (p=0.006, Student’s t-test). Pre-operatively the median rotation within the operated segment was 4.6°. -

Open Microlumbar Laminectomy & Discectomy
Open Microlumbar Laminectomy & Discectomy What is it? Open microlumbar laminotomy/discectomy is an operation performed on the lower spine to relieve pressure on one or more nerve roots. The operation is usually performed under general anesthesia or a spinal anesthetic. Frequently it is performed as an inpatient but if the patient is stoic and doctor agrees it can be performed as an outpatient procedure. A catheter may be placed in your bladder if the surgery is planned to take a long time. Why Is It Done? When an intervertebral disc ruptures in the lumbar spine, it puts pressure on one or more nerve roots (often called nerve root compression). This causes pain and other symptoms in the neck, arms, and even legs. In this operation, the surgeon reaches the lumbar spine through a small incision in the low back. After the muscles of the spine are stripped from the vertebral bone the nerve root is exposed by removing part of the bony covering (lamina), which covers the nerve root. This is called a laminotomy. Usually as much as possible of the abnormal intervertebral disc is then removed taking the pressure off of the nerve root. Since the spinal canal is opened there may be post-operative scar formation which can cause delayed recurrent nerve pain. Since much of the nuclear material is removed from the center of the disc, delayed vertical instability with disc space collapse frequently will occur. What Happens Afterwards? At home you will have to take narcotic medication to help with any discomfort. Any severe increase in pain not controlled with the medication should also be reported to your physician or the physician's nurse. -

Fusion Analysis on CT Spine Exams Thomas J
CLINICAL GUIDELINES Fusion Analysis on CT Spine Exams Thomas J. Gilbert M.D., M.P.P., Mark E. Myers M.D., and Damon J. Spitz M.D. 10/26/15 Introduction Multidetector computed tomography (MDCT) is a first line study in the evaluation of fusion patients. (Burkus, Epstein, Gruskay, Goldstein, Patel) CT is useful to evaluate for healing of a fusion although some authors advocate CT only in cases in which plain radiographic evaluation is indeterminate. (Buchowski, Rhee) It is important for the radiologist to evaluate the status of a spinal fusion. It is insufficient to merely report the presence of a fusion. A nonunion or pseudarthrosis may be a source of pain or may contribute to the patient’s symptoms. In addition, the integrity of a spine fusion is an important factor in surgical planning in patients undergoing fusion revision, adjacent segment disease or adjacent segment deformity. Multidetector Computed Tomography (MDCT) with sagittal and coronal reconstructions is the most accurate examination to assess to integrity of a fusion. (Burkus, Epstein, Gruskay, Goldstein, Patel) MDCT accurately displays bony anatomy, produces high resolution images of bony reconstructions and allows for direct visualization of the fusion bed. CT has limited soft tissue contrast however, and has limited ability to directly visualize neurologic structures. CT has difficulty differentiating post-operative fibrosis from recurrent disc herniation following dorsal decompression surgery and discectomy. CT also has difficulty accurately grading central and subarticular recess stenosis within or adjacent to a fusion. While the reported accuracy of CT for stenosis is similar to that with MRI, in practice, delineation of the dural sac within an area of central or subarticular stenosis can be difficult. -

Long-Term Clinical Outcomes After Bilateral Laminotomy Or Total Laminectomy for Lumbar Spinal Stenosis: a Single-Institution Experience
NEUROSURGICAL FOCUS Neurosurg Focus 46 (5):E2, 2019 Long-term clinical outcomes after bilateral laminotomy or total laminectomy for lumbar spinal stenosis: a single-institution experience Andrea Pietrantonio, MD,1,3 Sokol Trungu, MD,1,2 Isabella Famà, MD,1 Stefano Forcato, MD,1,2 Massimo Miscusi, MD, PhD,1 and Antonino Raco, MD, PhD1 1Department of Neuroscience, Mental Health, and Sense Organs, Faculty of Medicine and Psychology, ‘‘Sapienza” University of Rome, Sant’Andrea Hospital, Rome; 2Neurosurgery Unit, Cardinale G. Panico Hospital, Tricase; and 3Neurosurgery Unit, Santa Maria Goretti Hospital, Latina, Italy OBJECTIVE Lumbar spinal stenosis (LSS) is the most common spinal disease in the geriatric population, and is char- acterized by a compression of the lumbosacral neural roots from a narrowing of the lumbar spinal canal. LSS can result in symptomatic compression of the neural elements, requiring surgical treatment if conservative management fails. Dif- ferent surgical techniques with or without fusion are currently treatment options. The purpose of this study was to provide a description of the long-term clinical outcomes of patients who underwent bilateral laminotomy compared with total laminectomy for LSS. METHODS The authors retrospectively reviewed all the patients treated surgically by the senior author for LSS with to- tal laminectomy and bilateral laminotomy with a minimum of 10 years of follow-up. Patients were divided into 2 treatment groups (total laminectomy, group 1; and bilateral laminotomy, group 2) according to the type of surgical decompression. Clinical outcomes measures included the visual analog scale (VAS), the 36-Item Short-Form Health Survey (SF-36) scores, and the Oswestry Disability Index (ODI). -

SJH Procedures
SJH Procedures - Pain Management Service New Name Old Name CPT Code Service BLOCK, FACET JOINT, LUMBAR LUMBAR FACET NERVE BLOCK 64493 Injection(s), diagnostic or therapeutic agent, paravertebral Pain Management facet (zygapophyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), lumbar or sacral; single level 64494 Injection(s), diagnostic or therapeutic agent, paravertebral Pain Management facet (zygapophyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), lumbar or sacral; second level (List separately in addition to code for primary procedure) 64495 Injection(s), diagnostic or therapeutic agent, paravertebral Pain Management facet (zygapophyseal) joint (or nerves innervating that joint) with image guidance (fluoroscopy or CT), lumbar or sacral; third and any additional level(s) (List separately in addition to code f BLOCK, SPINAL NERVE ROOT, LUMBAR, TRANSFORAMINAL APPROACH LUMBAR TRANSFORAMINAL NERVE BLOCK 0230T Injection(s), anesthetic agent and/or steroid, transforaminal Pain Management epidural, with ultrasound guidance, lumbar or sacral; single level 64483 Injection(s), anesthetic agent and/or steroid, transforaminal Pain Management epidural, with imaging guidance (fluoroscopy or CT); lumbar or sacral, single level 64484 Injection(s), anesthetic agent and/or steroid, transforaminal Pain Management epidural, with imaging guidance (fluoroscopy or CT); lumbar or sacral, each additional level (List separately in addition to code for primary procedure) INJECTION,