Biological Recovery of the Bohemian Forest Lakes from Acidification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Potential European Bison (Bison Bonasus) Habitat in Germany
PROJECT REPORT Potential European bison (Bison bonasus) habitat in Germany Tobias Kuemmerle Humboldt-University Berlin Benjamin Bleyhl Humboldt-University Berlin Wanda Olech University of Warsaw & European Bison Friends Society Kajetan Perzanowski Carpathian Wildlife Research Station MIZ, PAS & European Bison Friends Society 1 PROJECT REPORT CONTENTS CONTENTS ...............................................................................................................................................................2 INTRODUCTION .......................................................................................................................................................3 HISTORIC DISTRIBUTION OF EUROPEAN BISON IN EUROPE .....................................................................................4 EUROPEAN BISON HABITAT PREFERENCES ..............................................................................................................6 HABITAT USE OF CONTEMPORARY EUROPEAN BISON POPULATIONS .......................................................................................... 6 PALEO-ECOLOGICAL DATA ON EUROPEAN BISON HABITAT USE ............................................................................................... 10 MAPPING EUROPEAN BISON HABITAT IN GERMANY ............................................................................................ 10 APPROACH ................................................................................................................................................................. -

Biodiversity of the Hercynian Mountains of Central Europe
Pirineos, 151-152: 83 a 99, JACA; 1998 BIODIVERSITY OF THE HERCYNIAN MOUNTAINS OF CENTRAL EUROPE JAN JENÍK Faculty of Science, Charles University, Benátská 2, CZ-12801 Praha 2, Czech Republic SUMMARY.- The vegetation of temperate Central Europe north of the Alps is mainly of low diversity broadleaf and conifer forest. The occurrence of three azonal habitat types: mires with their numerous microhabitats, the georelief of the karst and its deeply-cut river valleys, and ecological islands with a distinct vegetation near the treeline of the middle-mountains causes local areas of high diversity. These high species diversity spots are the result of an interplay between physical, biotic and historical factors. A model of an anemo-orographic system with its underlying factors is described to explain the high plant and animal diversity in the corries (glacial cirques) of the Hercynian mountains. RÉSUMÉ.-La végétation de l'Europe Centrale tempérée au nord des Alpes nous montre surtout des forêts à de feuillues et de connifères à faible diversité. Cependant, la présence de trois types d'habitats azonaux peut produire une haute diversité au niveau local: zones humides avec leur nombreux microhabitats, reliefs karstiques et leur profonds défilés fluviaux, et enfin des îles écologiques avec une végétation par ticulière situées près de la limite supérieure des arbres (treeline). Ces secteurs riches en espèces peuvent s'expliquer par l'interaction des facteurs physiques, biotiques et historiques. Dans ce domaine nous proposons un système anémo-orographique avec ses facteurs inféodés qui pourrait expliquer la haute diversité animale et végétale dans les cirques glaciaires des montagnes hercyniennes. -

Proxy Environmental Record from the Bohemian Forest, Czech Republic
bs_bs_banner The Lateglacial and Holocene in Central Europe: a multi-proxy environmental record from the Bohemian Forest, Czech Republic KLARA VOCADLOV A, LIBOR PETR, PAVLA ZA CKOV A, MAREK KRIZEK, LENKA KRIZOV A, SIMON M. HUTCHINSON AND MIROSLAV SOBR Vocadlova, K., Petr, L., Zackova, P., Krızek, M., Krızova, L., Hutchinson, S. M. & Sobr M.: The Lateglacial and Holocene in Central Europe: a multi-proxy environmental record from the Bohemian Forest, Czech Republic. Boreas. 10.1111/bor.12126. ISSN 0300-9483. The Hercynian mountain ranges were islands of mountain glaciation and alpine tundra in a Central European ice-free corridor during the Late Pleistocene. Today they are notable areas of glacial landforms, alpine-forest free areas, peatlands and woodlands. However, our knowledge of the Lateglacial and early Holocene environmental changes in this region is limited. We present a new multi-proxy reconstruction of a mid-altitude environment in the Bohemian Forest spanning this period. A core (5.2 m length) in the Cern e Lake cirque (1028 m a.s.l.) was sub- jected to lithological, geochemical, pollen and macrofossil analysis supplemented by two optically stimulated luminescence (OSL) and 10 AMS radiocarbon dates. We determined the impact of regional and supraregional cli- mate changes on the environment. The two most significant changes in sedimentation during the Lateglacial (17.6 and 15.8–15.5 cal. ka BP) were synchronous with regional glacial chronostratigraphy. Unlike Central European mountain ranges, in the Bohemian Forest the Younger Dryas was not coincident with glacier re-advance, but was a dry, cold episode with low lake levels, which prevailed until the early Preboreal. -

Sulphate Sorption Characteristics of the Bohemian Forest Soils
Silva Gabreta vol. 11 (1) p. 3–12 Vimperk, 2005 Sulphate sorption characteristics of the Bohemian Forest soils Jiří Kaňa* & Jiří Kopáček Faculty of Biological Sciences, University of South Bohemia, and Hydrobiological Institute, AS CR, Na Sádkách 7, CZ-37005 České Budějovice, Czech Republic *[email protected] Abstract Sulphate (SO4-S) sorption characteristics were determined in soils from catchments of three acidified mountain lakes, Plešné (PL), Čertovo (CT), and Černé (CN), situated in the Bohemian Forest. The SO4-S desorption ranged between 0.07–0.73, 0.21–0.68, and 0.39–0.82 mmol.kg–1 in the PL, CT, and CN catchments, respectively. SO4-S desorption was observed to be higher in horizon A than in mineral horizons below. The SO4-S represented only 3.4% of total S pool in the catchments and did not explain a relatively high rate of terrestrial leaching of sulphate over last 10 years. This disproportion suggests that the soil SO4-S pool is being replenished from internal soil processes from the pool of total S. Sulphate sorption maxima in soils in the catchments of the Bohemian Forest lakes varied between 1.5–11.7 mmol.kg–1, being highest in A-horizon and lowest in E-horizon. Key words: acidification, recovery, soil chemistry INTRODUCTION The chemistry (and, consequently, biology) of mountain lakes is determined by amount of precipitation and their composition, bedrock (PSENNER & CATALAN 1994), catchment soils and vegetation (e.g. BARON et al. 1994), and hydrology (WETZEL 2002). Soil sorption charac- teristics may significantly affect water composition, e.g. -

Vegetation of the Czech Republic: Diversity, Ecology, History and Dynamics
Preslia 84: 427–504, 2012 427 Vegetation of the Czech Republic: diversity, ecology, history and dynamics Vegetace České republiky: diverzita, ekologie, historie a dynamika Dedicated to the centenary of the Czech Botanical Society (1912–2012) Milan C h y t r ý Department of Botany and Zoology, Masaryk University, Kotlářská 2, CZ-611 37 Brno, Czech Republic, e-mail: [email protected] Chytrý M. (2012): Vegetation of the Czech Republic: diversity, ecology, history and dynamics. – Preslia 84: 427–504. This review summarizes basic information on the diversity of vegetation in the Czech Republic. It describes basic environmenal factors affecting vegetation, vegetation history since the last glacial, biomes occurring in the Czech Republic (zonal biomes of broad-leaved deciduous forest and forest- steppe, and azonal biomes of taiga and tundra), altitudinal zonation of vegetation and landscapes with an exceptionally high diversity of vegetation types (deep river valleys in the Bohemian Massif, karst areas, sandstone pseudokarst areas, solitary volcanic hills, glacial cirques, lowland riverine landscapes and serpentine areas). Vegetation types, delimited according to the monograph Vegeta- tion of the Czech Republic, are described with emphasis on their diversity, ecology, history and dynamics. K e y w o r d s: alpine, aquatic, central Europe, forest, grassland, phytosociology, plant communi- ties, ruderal, vegetation change, vegetation classification, vegetation history, weed, wetland Introduction The Czech Republic is a land-locked country in central Europe occupying an area of 78,867 km2. It is situated in the zone of temperate broad-leaved deciduous forest, which in the south-east borders on the forest-steppe zone. -

Eastern Bavaria
Basic text Eastern Bavaria Culture Eastern Bavaria is still home to more castles than anywhere else in Germany: Some medieval castles remain only as ruins, whilst other castles such as Falkenstein Castle have withstood decline and are open to visitors. The expansive spruce forests in Eastern Bavaria have given way to the Bavarian Glass Road, as they supplied the wood and quartz sand –the key raw materials – for the very first glass foundries. Spanning some 250 kilometres, it is one of the most picturesque holiday routes in Germany. Those choosing to travel along the route will learn all about the 700-year tradition of glass production and glass as a form of art. The route, which begins in Neustadt an der Waldnaab and leads to Passau, features glass foundries, galleries and museums, all packed to the brim with interesting facts about the traditional handicraft. Some Eastern Bavarian companies are keeping the tradition alive to this day and export to countries ranging from the United Arab Emirates to the United States of America. The largest towns in Eastern Bavaria include Regensburg, Landshut and Passau. The city of Regensburg, which was first founded by Roman Emperor Marcus Aurelius, has retained its medieval centre to this day. The Old Town of Regensburg together with Stadtamhof has been a UNESCO World Heritage Site since 2006. Landshut is the prototype of an old Bavarian town. Above all its town centre, which features gabled houses, decorative façades, oriels and arches, is one of the most beautiful squares to be found in the whole of Germany. The three-river town of Passau, which was built in the Italian baroque style, achieved early wealth thanks to its participation in the salt trade and was a place of border crossings due to its location on the border with Austria and just 30 kilometres from the Czech border. -
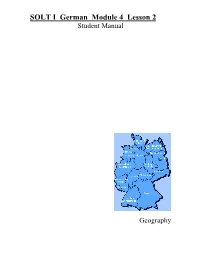
SOLT I German Module 4 Lesson 2 Student Manual
SOLT I German Module 4 Lesson 2 Student Manual Geography Geography German SOLT I Objectives Module 4 Lesson 2 At the end of this lesson you will be able identify the different German states and the countries surrounding Germany. In order to achieve this objective you will: Recognize the German States and Their Locations • Recognize the states’ locations, their capitals, major cities, and regions within the states • Describe their areas and borders • Brief about Germany • Describe the geography of Germany • Talk about the location of military installations in Germany • Identify the national flag of the country Identify Nationalities • Identify languages in the world countries • Identify different ethnic groups • Describe demographic composition 47 Geography German SOLT I Introduction Module 4 Lesson 2 Features of Germany You are planning a short vacation somewhere in Germany and have asked one of your German counterparts to describe some of the various landscapes, since you can’t decide. He gives you a quick description of Germany’s geography. Where will you decide to go? Deutschland hat sechzehn Bundesländer. Die Hauptstadt von Deutschland ist Berlin. In jedem Bundesland gibt es sehr schöne Landschaften. Im Norden ist die Nordsee mit der schönen Insel Sylt. Das Land ist flach. Die Bundesländer dort sind Schleswig-Holstein, Hamburg und Bremen. In der Mitte von Deutschland gibt es viele Berge. Besonders schön ist der Teutoburger Wald und der Hunsrück. Weiter südlich gibt es natürlich den berühmten Schwarzwald im Westen und den Bayrischen Wald. Einige Bundesländer dort sind Sachsen-Anhalt, Thüringen, Hessen und Nordrhein-Westfalen. Im südlichen Teil von Deutschland liegt Bayern. -

Towards a Dendrochronologically Refined Date of the Laacher See
Quaternary Science Reviews 229 (2020) 106128 Contents lists available at ScienceDirect Quaternary Science Reviews journal homepage: www.elsevier.com/locate/quascirev Towards a dendrochronologically refined date of the Laacher See eruption around 13,000 years ago * Frederick Reinig a, b, , Paolo Cherubini a, c, Stefan Engels d, Jan Esper b, Giulia Guidobaldi a, Olaf Joris€ e, f, g, Christine Lane h, Daniel Nievergelt a, Clive Oppenheimer h, Cornelia Park i, Hardy Pfanz j, Felix Riede k, Hans-Ulrich Schmincke i, Martin Street e, Lukas Wacker l, Ulf Büntgen a, h, m, n a Swiss Federal Research Institute WSL, Birmensdorf, Switzerland b Department of Geography, Johannes Gutenberg University, Mainz, Germany c Department of Forest and Conservation Sciences, University of British Columbia, Vancouver, BC, Canada d Department of Geography, Birkbeck University of London, London, UK e MONREPOS Archaeological Research Centre and Museum for Human Behavioural Evolution, Neuwied, Germany f Department of Prehistoric and Protohistoric Archaeology, Johannes Gutenberg University, Mainz, Germany g MOE Key Laboratory of Western China’s Environmental Systems Research School of Arid Environment and Climate Change, Lanzhou, China h Department of Geography, University of Cambridge, Cambridge, UK i GEOMAR Helmholtz Centre for Ocean Research Kiel, Germany j Institut für Angewandte Botanik und Vulkanbiologie, Universitat€ Duisburg-Essen, Essen, Germany k Department of Archaeology and Heritage Studies, Aarhus University, Højbjerg, Denmark l ETH Zurich, Laboratory of Ion Beam Physics, Zurich, Switzerland m Global Change Research Institute of the Czech Academy of Sciences (CzechGlobe), Brno, Czech Republic n Department of Geography, Faculty of Science, Masaryk University, Brno, Czech Republic article info abstract Article history: The precise date of the Laacher See eruption (LSE), central Europe’s largest Late Pleistocene volcanic Received 13 May 2019 event that occurred around 13,000 years ago, is still unknown. -

Changes in Forest Structure in the Bavarian Forest National Park – an Evaluation After 10 Years of the BIOKLIM-Project
Silva Gabreta vol. 24 p. 161–170 Vimperk, 2018 Changes in forest structure in the Bavarian Forest National Park – an evaluation after 10 years of the BIOKLIM-Project Torben Hilmers1,*, Claus Bässler2, Nicolas Friess3, Marco Heurich2, Jörg Müller2,4 & Linda Seifert2 1Technical University of Munich, Hans-Carl-Von-Carlowitz-Platz 2, D-85354 Freising, Germany 2Bavarian Forest National Park, Freyunger Str. 2, D-94481 Grafenau, Germany 3Faculty of Biology, Philipps-Universität Marburg, Karl-von-Frisch Str. 8, D-35032 Marburg, Germany 4University of Würzburg, Glashüttenstr. 5, D-96181 Rauhenebrach, Germany * [email protected] Abstract Biodiversity is diminishing globally at an unprecedented rate in times of intensive land use and ongo- ing climate change. Since biodiversity is related to important ecosystem functions and services it is nowadays the goal by law to maintain and improve biodiversity. In this perspective, the BIOKLIM- Project carried out two large forest structure and biodiversity surveys within the Bavarian Forest National Park in 2006 and 2016 to provide a broad range of data to assess the effects of a changing forest structure and climate on species and communities across different taxonomic groups. In this paper, we present the changes in forest structure between the two surveys. Results showed that study plots which were formerly affected from disruptive events such as storms and bark beetle infestations made progress in succession and thus occupy higher classes of forest succession. Furthermore, the results showed that disruptive events again caused disturbances, especially at the high elevations of the Bavarian Forest National Park. Hence, disturbances mainly affected spruce. Anyway, since the forest systems, with the exception of disturbances that might occur, are inert systems, the forest structure changed only slightly on the study plots between the two years of investigation. -

Forest Edges in the Mixed-Montane Zone of the Bavarian Forest National Park – Hot Spots of Biodiversity
Silva Gabreta vol. 13 (2) p. 121–148 Vimperk, 2007 Forest edges in the mixed-montane zone of the Bavarian Forest National Park – hot spots of biodiversity Jörg Müller1,*, Heinz Bußler2, Martin Goßner3, Axel Gruppe4, Andrea Jarzabek- Müller1, Manuel Preis1 & Thomas Rettelbach5 1Bavarian Forest National Park, Freyunger Strasse 2, D-94481 Grafenau, Germany 2Bayerische Landesanstalt für Wald und Forstwirtschaft, Am Hochanger 13, D-85354 Freising, Germany 3Schussenstrasse 12, D-88273 Fronreute, Germany 4Department of Animal Ecology, Technical University Munich, Am Hochanger 13, D-85354 Freising, Germany 5Fritzenanger 9, D-83362 Surberg, Germany *[email protected] Abstract Using 60 flight interception traps we investigated twelve different forest edges in the National Park Bava- rian Forest. Half of the forest edges studied were created by bark beetle attacks (interior edges), the others result from clearance for farming by humans. At each forest edge, five traps were installed as follows: one trap was placed in the open area 50–80 m distant from the forest edge, two traps at the edge (one at ground level, one in the canopy), and two traps in the forest interior 70–100 m away from the edge (one at ground level, one in the canopy). We caught and determined 10,966 specimens representing 421 insect species: 240 beetles, 96 true bugs, 65 Aculeata, and 20 lacewings. The highest number of species was found in the open spaces and at the edges. Bark beetle gaps are shown as hot spots of insect biodiversity. Key words: interior forest edge, exterior forest edge, biodiversity, flight interception traps, bark beetles. -

Remote Sensing of Forest Decline in the Czech Republic
££ V807/5Z Remote Sensing of Forest Decline in theCzech Republic fiNBC6lVE0 m OST i ■■ '' Jonas Ardo MEDDELANDEN FRAN LUNDS UNIVERSITETS GEOGRAFISKAINSTITUTIONER avhandlingar 135 DISCLAIMER Portions of this document may be illegible electronic image products. Images are produced from the best available original document. MEDDELANDEN FRAN LUNDS UNIVERSITETS GEOGRAFISKA INSTITUTIONER Avhandlingar 135 Remote Sensing of Forest Decline in the Czech Republic Jonas Ardo 1998 LUND UNIVERSITY, SWEDEN DEPARTMENT OF PHYSICAL GEOGRAPHY Author's address: Department of Physical Geography Lund University Box 118, S-221 00 Lund Sweden Email: [email protected] URL: www.natgeo.lu.se Lund University Press Box 141 S-221 00 Lund Sweden Art nr 20532 ISSN 0346-6787 ISBN 91-79-66-528-4 © 1998 Jonas Ardo Printed by KFS AB, Lund, Sweden 1998. Remote Sensing of Forest Decline in the Czech Republic Jonas Ardo N aturgeografiska institutional! Matematisk-naturvetenskapligfakultet Lunds universitet, Lund Avhandling FOR FILOSOFIE DOKTORSEXAMEN som kommer att offentligen forsvaras i Geografiska institutionemas forelasningssal, 3:e vaningen, Solvegatan 13, Lund, onsdagen den 20 maj 1998, kl. 13.15 Fakultetsopponent: Professor Curtis Woodcock, Department of Geography, Boston University, Boston, USA Document name LUND UNIVERSITY DOCTORAL DISSERTATION Physical Geography Date of issue Remote Sensing and GIS 1998-04-27 CODEN: ISRN lu : OTU3/NBNG-96/ 1135-SE Autbor(s) Sponsoring organisation Jonas Ardo Swedish National Space Board Title and subtitie Remote Sensing of Forest Decline in the Czech Republic This thesis describes the localisation and quantification of deforestation and forest damage in Norway spruce forests in northern Czech Republic using Landsat data. Severe defoliation increases the spectral reflectance in all wavelength bands, especially in the mid infrared region. -

Šumava – Bayerischer Wald/Unterer Inn – Mühlviertel
Crossing the borders. Studies on cross-border cooperation within the Danube Region The Euroregion Šumava – Bayerischer Wald/Unterer Inn – Mühlviertel Contents 1. Introduction ......................................................................................................................... 2 2. Development of cross-border cooperation .......................................................................... 5 2.1 The development of the Euroregion Šumava-Bayerischer Wald/Unterer Inn- Mühlviertel ..................................................................................................................... 6 3. Geographical characteristics of the area .............................................................................. 8 4. Organisational and institutional structures, activities ......................................................... 16 4.1 The emergence of the institution as a single cross-border unit ................................ 16 4.2 Spatial definition and membership in the Euroregion ............................................... 17 4.3 Organisational structure .............................................................................................. 19 4.4 Main activities .............................................................................................................. 21 5. Composition of the working group ..................................................................................... 23 6. Main activity fields ............................................................................................................