Blue-Eye Cichlid (Cryptoheros Spilurus) Ecological Risk Screening Summary
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two New Species of Australoheros (Teleostei: Cichlidae), with Notes on Diversity of the Genus and Biogeography of the Río De La Plata Basin
Zootaxa 2982: 1–26 (2011) ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2011 · Magnolia Press ISSN 1175-5334 (online edition) Two new species of Australoheros (Teleostei: Cichlidae), with notes on diversity of the genus and biogeography of the Río de la Plata basin OLDŘICH ŘÍČAN1, LUBOMÍR PIÁLEK1, ADRIANA ALMIRÓN2 & JORGE CASCIOTTA2 1Department of Zoology, Faculty of Science, University of South Bohemia, Branišovská 31, 370 05, České Budějovice, Czech Republic. E-mail: [email protected], [email protected] 2División Zoología Vertebrados, Facultad de Ciencias Naturales y Museo, UNLP, Paseo del Bosque, 1900 La Plata, Argentina. E-mail: [email protected], [email protected] Abstract Two new species of Australoheros Říčan and Kullander are described. Australoheros ykeregua sp. nov. is described from the tributaries of the río Uruguay in Misiones province, Argentina. Australoheros angiru sp. nov. is described from the tributaries of the upper rio Uruguai and middle rio Iguaçu in Brazil. The two new species are not closely related, A. yke- regua is the sister species of A. forquilha Říčan and Kullander, while A. angiru is the sister species of A. minuano Říčan and Kullander. The diversity of the genus Australoheros is reviewed using morphological and molecular phylogenetic analyses. These analyses suggest that the described species diversity of the genus in the coastal drainages of SE Brazil is overestimated and that many described species are best undestood as representing cases of intraspecific variation. The dis- tribution patterns of Australoheros species in the Uruguay and Iguazú river drainages point to historical connections be- tween today isolated river drainages (the lower río Iguazú with the arroyo Urugua–í, and the middle rio Iguaçu with the upper rio Uruguai). -

Hemichromis Bimaculatus (African Jewelfish)
African Jewelfish (Hemichromis bimaculatus) Ecological Risk Screening Summary U.S. Fish and Wildlife Service, April 2011 Revised, September 2018 Web Version, 2/14/2019 Photo: Zhyla. Licensed under CC BY-SA 3.0. Available: https://commons.wikimedia.org/wiki/File:Hemichromis_bimaculatus1.jpg. (September 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2018): “Africa: widely distributed in West Africa, where it is known from most hydrographic basins [Teugels and Thys van den Audenaerde 2003], associated with forested biotopes [Daget and Teugels 1991, Lamboj 2004]. Also reported from coastal basins of Cameroon, Democratic Republic of the Congo and Nile basin [Teugels and Thys van den Audenaerde 1992], but at least its presence in Cameroon is unconfirmed in [Stiassny et al. 2008]. [Lamboj 2004] limits this species to Guinea, Sierra Leone and Liberia.” 1 From Azeroual and Lalèyè (2010): “This species is widely distributed throughout western Africa, but has also been recorded from Algeria to Egypt.” “Northern Africa: Within this region this species is very rare. It used to be caught from the coastal lagoons, especially Lake Mariut (Egypt) and Algeria. Its [sic] found in Tunisia in the wadis of Kebili in the south of Tunisia and in wadis near Chott Melrhir in eastern Algeria (Kraiem, pers. comm.), and Egypt (Wadi El Rayan Lakes).” “Western Africa: It is known from most hydrographic basins in western Africa.” Status in the United States It is not certain if this species is present in the United States, or if records pertain to H. letourneuxi. From NatureServe (2018): “Introduced and established in Dade County, Florida, […] (Nelson 1983).” From Nico et al. -

They're Not Just Convicts Anymore
2008 FAAS Publication Awards. Please follow reprint instructions at http://www.faas.info/2008_publication_awards_winners.html#reprintpolicy They’re Not Just Convicts Anymore By Daniel Spielman All Photos by Daniel Spielman Introduction Convicts get no respect, with many a cichlidophile turning up his or her nose at the sight of a group on Convicts in a tank or a bag of fry in an auction. It’s time for that to change. Easy to breed and exhibiting wonderful parental care, Convict cichlids (Cryptoheros nigrofasciatus) have long been staples in the hobby. Indeed, for many new fishkeepers, the satisfaction of watching a pair of Convict parents herd a group of fry around a tank sparks the initial desire to keep other mem bers of the cichlid family. Indeed, my fish cichlids were Convicts, and watch ing them care for their fry definitely got me hooked. The contrast with trying to keep guppies or swordtails from eating their own offspring is striking (how did eating one’s own young ever evolve in the first place?). However, the very fact that Convicts spawn so readily in the aquarium causes many hobbyists to quickly lose interest in the species. Although Convicts occasionally appear on experienced hobbyists’ lists of most favorite cichlid, the problem is they are just too common and too easy to breed. Typical Convict spawning jokes involve two fish and a wet paper towel, and one often feels fortunate to break the $1 barrier at the auction to avoid the indignity of having to bring one’s fish back home again at the end of the monthly club meeting. -

Neue Gattungseinteilung Der Mittelamerikanischen Cichliden
DCG_Info_07_2016_HR_20160621_DCG_Info 21.06.2016 06:51 Seite 146 Neue Gattungseinteilung der mittelamerikanischen Cichliden Rico Morgenstern Foto: Juan Miguel Artigas Azas Theraps irregularis verbleibt als einzige Art in der Gattung Theraps. Die Aufnahme entstand im Rio Lacanja im südlichen Chiapas, Mexiko. Inzwischen ist es fast 33 Jahre her, wenigstens Versuche, einzelne Gat- schien die Abhandlung „Diversity and dass KULLANDER (1983) die Gattung tungen neu zu definieren – aber eine evolution of the Middle American cich- Cichlasoma auf zwölf südamerikani- umfassende Gesamtbearbeitung er- lid fishes (Teleostei: Cichlidae) with re- sche, nahe mit der Typusart C. bima- folgte bisher nicht. Vielfach wurden vised classification“ (ŘIČAN et al. culatum verwandte Arten beschränkte. Zuordnungen vorgenommen, ohne 2016). Seither durfte der Name streng ge- dass man sich um eine wirkliche Be- nommen für die Mehrzahl der bis gründung bemühte. Die Arbeit berücksichtigt alle Cichliden dahin in dieser ehemaligen Sammel- Nord- und Mittelamerikas und der An- gattung untergebrachten, überwie- Bei der Gattungseinteilung der mittel- tillen sowie einige eng verwandte süd- gend mittelamerikanischen Arten amerikanischen Cichliden herrschte amerikanische Gattungen (Australoheros, nicht mehr verwendet werden. Man- somit bis vor kurzem ein ziemliches Caquetaia, Heroina, Mesoheros). gels geeigneter Alternativen ist das Chaos. Nun ist jedoch das Ende der An- Diese Fische gehören zu den „heroinen aber dennoch geschehen, wobei der führungszeichen (mit einer Ausnahme) -

Systematics and Historical Biogeography of Greater Antillean Cichlidae
Molecular Phylogenetics and Evolution 39 (2006) 619–627 www.elsevier.com/locate/ympev Systematics and historical biogeography of Greater Antillean Cichlidae Prosanta Chakrabarty ¤ University of Michigan Museum of Zoology, Fish Division, 1109 Geddes Avenue, Ann Arbor, MI 48109-1079, USA Received 15 July 2005; revised 8 January 2006; accepted 11 January 2006 Available online 21 February 2006 Abstract A molecular phylogenetic analysis recovers a pattern consistent with a drift vicariance scenario for the origin of Greater Antillean cichlids. This phylogeny, based on mitochondrial and nuclear genes, reveals that clades on diVerent geographic regions diverged concur- rently with the geological separation of these areas. Middle America was initially colonized by South American cichlids in the Cretaceous, most probably through the Cretaceous Island Arc. The separation of Greater Antillean cichlids and their mainland Middle American rel- atives was caused by a drift vicariance event that took place when the islands became separated from Yucatan in the Eocene. Greater Antillean cichlids are monophyletic and do not have close South American relatives. Therefore, the alternative hypothesis that these cich- lids migrated via an Oligocene landbridge from South America is falsiWed. A marine dispersal hypothesis is not employed because the drift vicariance hypothesis is better able to explain the biogeographic patterns, both temporal and phylogenetic. © 2006 Elsevier Inc. All rights reserved. Keywords: Cichlidae; Caribbean geology; Greater Antilles biogeography; Molecular systematics “The geology is in many respects uncertain, the phyletic 1999). The other category suggests Middle American ori- analysis inadequate and the fossil record wretched. We gins from a period of coalescence between these islands and have if not the worst case scenario deWnitely a very bad Yucatan in the early Cenozoic (Pitman et al., 1993; Pindell, one.” 1994; updated from Malfait and Dinkelman, 1972; Ted- ford, 1974). -

Phylogenetic Perspectives in the Evolution of Parental Care in Ray-Finned Fishes
Evolution, 59(7), 2005, pp. 1570±1578 PHYLOGENETIC PERSPECTIVES IN THE EVOLUTION OF PARENTAL CARE IN RAY-FINNED FISHES JUDITH E. MANK,1,2 DANIEL E. L. PROMISLOW,1 AND JOHN C. AVISE1 1Department of Genetics, University of Georgia, Athens, Georgia 30602 2E-mail: [email protected] Abstract. Among major vertebrate groups, ray-®nned ®shes (Actinopterygii) collectively display a nearly unrivaled diversity of parental care activities. This fact, coupled with a growing body of phylogenetic data for Actinopterygii, makes these ®shes a logical model system for analyzing the evolutionary histories of alternative parental care modes and associated reproductive behaviors. From an extensive literature review, we constructed a supertree for ray-®nned ®shes and used its phylogenetic topology to investigate the evolution of several key reproductive states including type of parental care (maternal, paternal, or biparental), internal versus external fertilization, internal versus external gestation, nest construction behavior, and presence versus absence of sexual dichromatism (as an indicator of sexual selection). Using a comparative phylogenetic approach, we critically evaluate several hypotheses regarding evolutionary pathways toward parental care. Results from maximum parsimony reconstructions indicate that all forms of parental care, including paternal, biparental, and maternal (both external and internal to the female reproductive tract) have arisen repeatedly and independently during ray-®nned ®sh evolution. The most common evolutionary transitions were from external fertilization directly to paternal care and from external fertilization to maternal care via the intermediate step of internal fertilization. We also used maximum likelihood phylogenetic methods to test for statistical correlations and contingencies in the evolution of pairs of reproductive traits. -

View/Download
CICHLIFORMES: Cichlidae (part 6) · 1 The ETYFish Project © Christopher Scharpf and Kenneth J. Lazara COMMENTS: v. 6.0 - 18 April 2020 Order CICHLIFORMES (part 6 of 8) Family CICHLIDAE Cichlids (part 6 of 7) Subfamily Cichlinae American Cichlids (Acarichthys through Cryptoheros) Acarichthys Eigenmann 1912 Acara (=Astronotus, from acará, Tupí-Guaraní word for cichlids), original genus of A. heckelii; ichthys, fish Acarichthys heckelii (Müller & Troschel 1849) in honor of Austrian ichthyologist Johann Jakob Heckel (1790-1857), who proposed the original genus, Acara (=Astronotus) in 1840, and was the first to seriously study cichlids and revise the family Acaronia Myers 1940 -ia, belonging to: Acara (=Astronotus, from acará, Tupí-Guaraní word for cichlids), original genus of A. nassa [replacement name for Acaropsis Steindachner 1875, preoccupied by Acaropsis Moquin-Tandon 1863 in Arachnida] Acaronia nassa (Heckel 1840) wicker basket or fish trap, presumably based on its local name, Bocca de Juquia, meaning “fish trap mouth,” referring to its protractile jaws and gape-and-suck feeding strategy Acaronia vultuosa Kullander 1989 full of facial expressions or grimaces, referring to diagnostic conspicuous black markings on head Aequidens Eigenmann & Bray 1894 aequus, same or equal; dens, teeth, referring to even-sized teeth of A. tetramerus, proposed as a subgenus of Astronotus, which has enlarged anterior teeth Aequidens chimantanus Inger 1956 -anus, belonging to: Chimantá-tepui, Venezuela, where type locality (Río Abácapa, elevation 396 m) is -
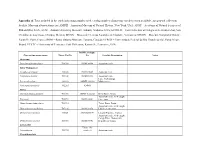
Appendix A. Taxa Included in the Study Indicating Samples Used, Catalog Number of Museum Vouchers When Available, and General Collection Locality
Appendix A. Taxa included in the study indicating samples used, catalog number of museum vouchers when available, and general collection locality. Museum abbreviations are: AMNH – American Museum of Natural History, New York, USA; ANSP – Academy of Natural Sciences of Philadelphia, USA; AUM – Auburn University Museum, Auburn, Alabama, USA; ECOSUR – Fish Collection at Colegio de la Frontera Sur, San Cristóbal de Las Casas, Chiapas, Mexico; MCNG – Museo de Ciencias Naturales de Guanare, Venezuela; MNHN – Muséum National d’Histoire Naturelle, Paris, France; ROM – Royal Ontario Museum, Toronto, Canada; UFRGS – Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil; UTFTC – University of Tennessee Fish Collection, Knoxville, Tennessee, USA. ROM Catalogue Current taxonomy name Tissue Cat No No Locality Description Notes Outgroup Pseudetroplus maculatus T14743 ROM 98998 Aquarium trade India-Madagascar Etroplus suratensis T13505 ROM 93809 Aquarium trade Paratilapia polleni T13100 ROM 88333 Aquarium trade Lake Andrapongy, Paretroplus damii 201936 AMNH 201936 Madagascar Paretroplus polyactis T12265 AMNH Africa Chromidotilapia guntheri T11700 AMNH I-226361 Beffa River, Benin Aquarium trade, wild caught, Etia nguti T10792 ROM 88042 Cameroon Hemichromis bimaculatus T11719 Tchan Duga, Benin Aquarium trade, wild caught, Heterochromis multidens T07136 ROM 88350 Lobeke, Cameroon Oreochromis niloticus 9092S AMNH254194 Littoral Province, Guinea Aquarium trade, wild caught, Congo River, Democratic Orthochromis stormsi T10766 ROM 88041 Republic of -

Mate Choice in Female Convict Cichlids (Amatitlania Nigrofasciata) and the Relationship Between Male Size and Dominance
J Ethol (2009) 27:249–254 DOI 10.1007/s10164-008-0111-2 ARTICLE Mate choice in female convict cichlids (Amatitlania nigrofasciata) and the relationship between male size and dominance Jennifer Gagliardi-Seeley Æ Joseph Leese Æ Nick Santangelo Æ M. Itzkowitz Received: 1 February 2008 / Accepted: 16 July 2008 / Published online: 21 August 2008 Ó Japan Ethological Society and Springer 2008 Abstract We examined how male size and fighting Keywords Mate choice Á Mate competition Á ability influence a female’s mate assessment process and Intrasexual competition Á Monogamy Á Cichlidae her eventual mate choice in the monogamous convict cichlid, Amatitlania nigrofasciata. Females always chose the larger of two males when they were allowed to see a Introduction larger male next to a smaller one and when a larger male defeated a smaller one in a fight. They did not differentiate In many systems, intra-sexually selected traits are typically between large and small males when they did not see the reinforced by inter-sexual selection. That is, the traits which two males together nor did they choose a dominant over a allow an individual to out-compete their own sex for access subordinate male when both were the same size. We sug- to resources are the same traits that attract mates (reviewed gest that females select on the basis of male size because it in Andersson 1994). Size is often such a trait; large indi- is a better predictor of both direct and indirect benefits (i.e., viduals out-compete small ones for resources and large future competitive interactions and foraging ability) than individuals are typically viewed as more attractive by the dominance behavior only. -

Systematic List of the Romanian Vertebrate Fauna
Travaux du Muséum National d’Histoire Naturelle © Décembre Vol. LIII pp. 377–411 «Grigore Antipa» 2010 DOI: 10.2478/v10191-010-0028-1 SYSTEMATIC LIST OF THE ROMANIAN VERTEBRATE FAUNA DUMITRU MURARIU Abstract. Compiling different bibliographical sources, a total of 732 taxa of specific and subspecific order remained. It is about the six large vertebrate classes of Romanian fauna. The first class (Cyclostomata) is represented by only four species, and Pisces (here considered super-class) – by 184 taxa. The rest of 544 taxa belong to Tetrapoda super-class which includes the other four vertebrate classes: Amphibia (20 taxa); Reptilia (31); Aves (382) and Mammalia (110 taxa). Résumé. Cette contribution à la systématique des vertébrés de Roumanie s’adresse à tous ceux qui sont intéressés par la zoologie en général et par la classification de ce groupe en spécial. Elle représente le début d’une thème de confrontation des opinions des spécialistes du domaine, ayant pour but final d’offrir aux élèves, aux étudiants, aux professeurs de biologie ainsi qu’à tous ceux intéressés, une synthèse actualisée de la classification des vertébrés de Roumanie. En compilant différentes sources bibliographiques, on a retenu un total de plus de 732 taxons d’ordre spécifique et sous-spécifique. Il s’agît des six grandes classes de vertébrés. La première classe (Cyclostomata) est représentée dans la faune de Roumanie par quatre espèces, tandis que Pisces (considérée ici au niveau de surclasse) l’est par 184 taxons. Le reste de 544 taxons font partie d’une autre surclasse (Tetrapoda) qui réunit les autres quatre classes de vertébrés: Amphibia (20 taxons); Reptilia (31); Aves (382) et Mammalia (110 taxons). -

BMC Evolutionary Biology Biomed Central
BMC Evolutionary Biology BioMed Central Research article Open Access Mitogenomic evaluation of the historical biogeography of cichlids toward reliable dating of teleostean divergences Yoichiro Azuma1, Yoshinori Kumazawa*2,3, Masaki Miya4, Kohji Mabuchi1 and Mutsumi Nishida1 Address: 1Ocean Research Institute, The University of Tokyo, 1-15-1 Minamidai, Nakano-ku, Tokyo 164-8639, Japan, 2Division of Material Science and Biological Science, Graduate School of Science, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8602, Japan, 3Department of Information and Biological Sciences, Graduate School of Natural Sciences, Nagoya City University, 1 Yamanohata, Mizuho-cho, Mizuho-ku, Nagoya 467-8501, Japan and 4Department of Zoology, Natural History Museum and Institute, Chiba, 955-2 Aoba-cho, Chuo-ku, Chiba 260-8682, Japan Email: Yoichiro Azuma - [email protected]; Yoshinori Kumazawa* - [email protected]; Masaki Miya - [email protected]; Kohji Mabuchi - [email protected]; Mutsumi Nishida - [email protected] * Corresponding author Published: 23 July 2008 Received: 18 March 2008 Accepted: 23 July 2008 BMC Evolutionary Biology 2008, 8:215 doi:10.1186/1471-2148-8-215 This article is available from: http://www.biomedcentral.com/1471-2148/8/215 © 2008 Azuma et al; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Abstract Background: Recent advances in DNA sequencing and computation offer the opportunity for reliable estimates of divergence times between organisms based on molecular data. -

Cichlasoma Bimaculatum) Ecological Risk Screening Summary
Black Acara (Cichlasoma bimaculatum) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2011 Revised, August 2014 and January 2018 Web Version, 4/5/2018 Photo: Florida Fish and Wildlife Conservation Commission. Licensed under Creative Commons (CC BY-NC). Available: https://www.invasive.org/browse/detail.cfm?imgnum=5371466. (January 2018). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2017): “South America: Orinoco River basin, in the Caroni […] River [in] Venezuela; Guianas, from the Essequibo River to the Sinnamary River; Amazon River basin, in the upper Branco River basin [Brazil].” 1 Status in the United States From Nico et al. (2018): “The species has been established in Florida since the early 1960s; it was first discovered in Broward County (Rivas 1965). The expanded geographic range of the species includes the counties of Broward (Courtenay et al. 1974; Courtenay and Hensley 1979a; museum specimens), Collier (Courtenay and Hensley 1979a; Courtenay et al. 1986; museum specimens), Glades (museum specimens), Hendry (Courtenay and Hensley 1979a; museum specimens), Highlands (Baber et al. 2002), Lee (Ceilley and Bortone 2000; Nico, unpublished), Martin (museum specimens), Miami-Dade (Kushlan 1972; Courtenay et al. 1974; Hogg 1976; Courtenay and Hensley 1979a; Loftus and Kushlan 1987; museum specimens), Monroe (Kushlan 1972; Courtenay et al. 1974; Courtenay and Hensley 1979a; Loftus and Kushlan 1987; museum specimens), Palm Beach (Courtenay et al. 1974; Courtenay and Hensley 1979a; museum specimens), Pasco (museum specimens), and Pinellas (museum specimens). It is established in Big Cypress National Preserve, Biscayne National Park, Everglades National Park (Kushlan 1972; Loftus and Kushlan 1987; Lorenz et al.