First Records of Cardiocondyla Ants from Myanmar
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
![Myrmicinae: Crematogastrini] Dacatria Rigato, 1994B: 155](https://docslib.b-cdn.net/cover/6356/myrmicinae-crematogastrini-dacatria-rigato-1994b-155-86356.webp)
Myrmicinae: Crematogastrini] Dacatria Rigato, 1994B: 155
DACATRIA [Myrmicinae: Crematogastrini] Dacatria Rigato, 1994b: 155. Type-species: Dacatria templaris Rigato, 1994b: 157, by original designation. Taxonomic history Dacatria in Myrmicinae, Proattini: Rigato, 1994b: 161. Dacatria in Myrmicinae, Stenammini: Bolton, 1994: 106; Bolton, 1995b: 26; Bolton, 2003: 59, 203. Dacatria in Myrmicinae, Crematogastrini: Ward, et al. 2015: 77; in Crematogastrini, Mayriella genus group: Blaimer, et al. 2018: 7. Dacatria as genus: all authors. Dacatria references: Eguchi, et al. 2011: 15 (diagnosis, Vietnam synopsis). DACETINOPS [Myrmicinae: Crematogastrini] Dacetinops Brown & Wilson, 1957a: 1. Type-species: Dacetinops cibdela Brown & Wilson, 1957: 4, by original designation. Taxonomic history Dacetinops in Myrmicinae, Leptothoracini: Wheeler, G.C. & Wheeler, J. 1985: 257. Dacetinops incertae sedis in Myrmicinae: Dlussky & Fedoseeva, 1988: 80. Dacetinops in Myrmicinae, Stenammini: Bolton, 1994: 106; Bolton, 1995b: 26; Bolton, 2003: 59, 203. Dacetinops in Myrmicinae, Crematogastrini: Ward, et al. 2015: 77; in Crematogastrini, Vollenhovia genus group: Blaimer, et al. 2018: 7. Dacetinops as genus: all authors. Dacetinops catalogues: Bolton, 1995b: 168. Dacetinops references: Taylor, 1985: 49 (all species revision, key). DACETON [Myrmicinae: Attini] Daceton Perty, 1833: 136. Type-species: Formica armigera Latreille, 1802c: 244, by monotypy. Taxonomic history Daceton in Poneridae, Myrmicidae: Smith, F. 1858b: 160. Daceton in Myrmicidae, Cryptoceridae: Smith, F. 1853: 226; Emery, 1877a: 81. Daceton in Cryptoceridae, Dacetini: Ashmead, 1905b: 384 [Dacetonini]. Daceton in Myrmicinae: Mayr, 1865: 26 [Myrmicidae]; Dalla Torre, 1893: 149 [Myrmicinae]. Daceton in Myrmicinae, Dacetini, Dacetiti: Brown, 1952g: 10 (footnote); Brown, 1954b: 465; Brown & Wilson, 1959b: 281; Brandão, 1991: 391. Daceton in Myrmicinae, Dacetini: Forel, 1893a: 164; Forel, 1892d: 344; Forel, 1895b: 136; Emery, 1895j: 770; Emery, 1896e: 180; Wheeler, W.M. -

ORIGINAL ARTICLE a Novel Intracellular Mutualistic Bacterium in the Invasive Ant Cardiocondyla Obscurior
The ISME Journal (2016) 10, 376–388 © 2016 International Society for Microbial Ecology All rights reserved 1751-7362/16 www.nature.com/ismej ORIGINAL ARTICLE A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior Antonia Klein1,8, Lukas Schrader1,8, Rosario Gil2, Alejandro Manzano-Marín2, Laura Flórez3, David Wheeler5, John H Werren6, Amparo Latorre2,7, Jürgen Heinze1, Martin Kaltenpoth3,4, Andrés Moya2 and Jan Oettler1 1Institut für Zoologie, Universität Regensburg, Regensburg, Germany; 2Institut Canvanilles de Biodiversitat i Biologia Evolutiva (ICBiBE), Parc Cientific de la Universitat de Valencia, Paterna (Valencia), Spain; 3Max Planck Institute for Chemical Ecology, Jena, Germany; 4Johannes Gutenberg University Mainz, Institute for Zoology, Department for Evolutionary Ecology, Mainz, Germany; 5Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand; 6Department of Biology, University Rochester, Rochester, NY, USA and 7Área de Genómica y Salud de la Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO)–Salud Pública, Valencia, Spain The evolution of eukaryotic organisms is often strongly influenced by microbial symbionts that confer novel traits to their hosts. Here we describe the intracellular Enterobacteriaceae symbiont of theinvasiveantCardiocondyla obscurior, ‘Candidatus Westeberhardia cardiocondylae’. Upon metamorphosis, Westeberhardia is found in gut-associated bacteriomes that deteriorate following eclosion. Only queens maintain Westeberhardia in the ovarian nurse cells from where the symbionts are transmitted to late-stage oocytes during nurse cell depletion. Functional analyses of the streamlined genome of Westeberhardia (533 kb, 23.41% GC content) indicate that neither vitamins nor essential amino acids are provided for the host. However, the genome encodes for an almost complete shikimate pathway leading to 4-hydroxyphenylpyruvate, which could be converted into tyrosine by the host. -

James K. Wetterer
James K. Wetterer Wilkes Honors College, Florida Atlantic University 5353 Parkside Drive, Jupiter, FL 33458 Phone: (561) 799-8648; FAX: (561) 799-8602; e-mail: [email protected] EDUCATION UNIVERSITY OF WASHINGTON, Seattle, WA, 9/83 - 8/88 Ph.D., Zoology: Ecology and Evolution; Advisor: Gordon H. Orians. MICHIGAN STATE UNIVERSITY, East Lansing, MI, 9/81 - 9/83 M.S., Zoology: Ecology; Advisors: Earl E. Werner and Donald J. Hall. CORNELL UNIVERSITY, Ithaca, NY, 9/76 - 5/79 A.B., Biology: Ecology and Systematics. UNIVERSITÉ DE PARIS III, France, 1/78 - 5/78 Semester abroad: courses in theater, literature, and history of art. WORK EXPERIENCE FLORIDA ATLANTIC UNIVERSITY, Wilkes Honors College 8/04 - present: Professor 7/98 - 7/04: Associate Professor Teaching: Biodiversity, Principles of Ecology, Behavioral Ecology, Human Ecology, Environmental Studies, Tropical Ecology, Field Biology, Life Science, and Scientific Writing 9/03 - 1/04 & 5/04 - 8/04: Fulbright Scholar; Ants of Trinidad and Tobago COLUMBIA UNIVERSITY, Department of Earth and Environmental Science 7/96 - 6/98: Assistant Professor Teaching: Community Ecology, Behavioral Ecology, and Tropical Ecology WHEATON COLLEGE, Department of Biology 8/94 - 6/96: Visiting Assistant Professor Teaching: General Ecology and Introductory Biology HARVARD UNIVERSITY, Museum of Comparative Zoology 8/91- 6/94: Post-doctoral Fellow; Behavior, ecology, and evolution of fungus-growing ants Advisors: Edward O. Wilson, Naomi Pierce, and Richard Lewontin 9/95 - 1/96: Teaching: Ethology PRINCETON UNIVERSITY, Department of Ecology and Evolutionary Biology 7/89 - 7/91: Research Associate; Ecology and evolution of leaf-cutting ants Advisor: Stephen Hubbell 1/91 - 5/91: Teaching: Tropical Ecology, Introduction to the Scientific Method VANDERBILT UNIVERSITY, Department of Psychology 9/88 - 7/89: Post-doctoral Fellow; Visual psychophysics of fish and horseshoe crabs Advisor: Maureen K. -

Borowiec Et Al-2020 Ants – Phylogeny and Classification
A Ants: Phylogeny and 1758 when the Swedish botanist Carl von Linné Classification published the tenth edition of his catalog of all plant and animal species known at the time. Marek L. Borowiec1, Corrie S. Moreau2 and Among the approximately 4,200 animals that he Christian Rabeling3 included were 17 species of ants. The succeeding 1University of Idaho, Moscow, ID, USA two and a half centuries have seen tremendous 2Departments of Entomology and Ecology & progress in the theory and practice of biological Evolutionary Biology, Cornell University, Ithaca, classification. Here we provide a summary of the NY, USA current state of phylogenetic and systematic 3Social Insect Research Group, Arizona State research on the ants. University, Tempe, AZ, USA Ants Within the Hymenoptera Tree of Ants are the most ubiquitous and ecologically Life dominant insects on the face of our Earth. This is believed to be due in large part to the cooperation Ants belong to the order Hymenoptera, which also allowed by their sociality. At the time of writing, includes wasps and bees. ▶ Eusociality, or true about 13,500 ant species are described and sociality, evolved multiple times within the named, classified into 334 genera that make up order, with ants as by far the most widespread, 17 subfamilies (Fig. 1). This diversity makes the abundant, and species-rich lineage of eusocial ants the world’s by far the most speciose group of animals. Within the Hymenoptera, ants are part eusocial insects, but ants are not only diverse in of the ▶ Aculeata, the clade in which the ovipos- terms of numbers of species. -

Invasive Ant Pest Risk Assessment Project: Preliminary Risk Assessment
Invasive ant pest risk assessment project: Preliminary risk assessment Harris, R. 1) Aim To assess the threat to New Zealand of a wide range of ant species not already established in New Zealand and identify those worthy of more detailed assessment. 2) Scope 2.1. Specific exclusions Solenopsis invicta was specifically excluded from consideration as this species has already been subject to detailed consideration by Biosecurity New Zealand. 2.2 Specific inclusions Biosecurity New Zealand requested originally that the following taxa be included in the assessment: Solenopsis richteri Solenopsis geminata Wasmannia auropunctata Anoplolepis gracilipes Paratrechina longicornis Carpenter ants (Camponotus spp.) Leaf cutting ants (Atta spp.) Myrmecia pilosula Tapinoma melanocephalum Monomorium sydneyense (incursion found in New Zealand) Hypoponera punctatissima (incursion found in New Zealand) Big headed ants (Pheidole spp.) M. sydneyense and H. punctatissima have since been deemed not under official control and are now considered established in New Zealand. Profiles of these species have been prepared as part of the Ants of New Zealand section (see http://www.landcareresearch.co.nz/research/biosecurity/stowaways/Ants/antsinnewzealand.asp). INVASIVE ANT PEST RISK ASSESSMENT PROJECT: Preliminary risk assessment 3) Methodology A risk assessment scorecard was developed (Appendix 1) in consultation with a weed risk assessment expert (Dr Peter Williams) and with Simon O’Connor and Amelia Pascoe of Biosecurity New Zealand, to initially separate -

ARTHROPODA Subphylum Hexapoda Protura, Springtails, Diplura, and Insects
NINE Phylum ARTHROPODA SUBPHYLUM HEXAPODA Protura, springtails, Diplura, and insects ROD P. MACFARLANE, PETER A. MADDISON, IAN G. ANDREW, JOCELYN A. BERRY, PETER M. JOHNS, ROBERT J. B. HOARE, MARIE-CLAUDE LARIVIÈRE, PENELOPE GREENSLADE, ROSA C. HENDERSON, COURTenaY N. SMITHERS, RicarDO L. PALMA, JOHN B. WARD, ROBERT L. C. PILGRIM, DaVID R. TOWNS, IAN McLELLAN, DAVID A. J. TEULON, TERRY R. HITCHINGS, VICTOR F. EASTOP, NICHOLAS A. MARTIN, MURRAY J. FLETCHER, MARLON A. W. STUFKENS, PAMELA J. DALE, Daniel BURCKHARDT, THOMAS R. BUCKLEY, STEVEN A. TREWICK defining feature of the Hexapoda, as the name suggests, is six legs. Also, the body comprises a head, thorax, and abdomen. The number A of abdominal segments varies, however; there are only six in the Collembola (springtails), 9–12 in the Protura, and 10 in the Diplura, whereas in all other hexapods there are strictly 11. Insects are now regarded as comprising only those hexapods with 11 abdominal segments. Whereas crustaceans are the dominant group of arthropods in the sea, hexapods prevail on land, in numbers and biomass. Altogether, the Hexapoda constitutes the most diverse group of animals – the estimated number of described species worldwide is just over 900,000, with the beetles (order Coleoptera) comprising more than a third of these. Today, the Hexapoda is considered to contain four classes – the Insecta, and the Protura, Collembola, and Diplura. The latter three classes were formerly allied with the insect orders Archaeognatha (jumping bristletails) and Thysanura (silverfish) as the insect subclass Apterygota (‘wingless’). The Apterygota is now regarded as an artificial assemblage (Bitsch & Bitsch 2000). -

Hymenoptera: Formicidae
16 The Weta 30: 16-18 (2005) Changes to the classification of ants (Hymenoptera: Formicidae) Darren F. Ward School of Biological Sciences, Tamaki Campus, Auckland University, Private Bag 92019, Auckland ([email protected]) Introduction This short note aims to update the reader on changes to the subfamily classification of ants (Hymenoptera: Formicidae). Although the New Zealand ant fauna is very small, these changes affect the classification and phylogeny of both endemic and exotic ant species in New Zealand. Bolton (2003) has recently proposed a new subfamily classification for ants. Two new subfamilies have been created, a revised status for one, and new status for four. Worldwide, there are now 21 extant subfamilies of ants. The endemic fauna of New Zealand is now classified into six subfamilies (Table 1), as a result of three subfamilies, Amblyoponinae, Heteroponerinae and Proceratiinae, being split from the traditional subfamily Ponerinae. Bolton’s (2003) classification also affects several exotic species in New Zealand. Three species have been transferred from Ponerinae: Amblyopone australis to Amblyoponinae, and Rhytidoponera chalybaea and R. metallica to Ectatomminae. Currently there are 28 exotic species in New Zealand (Table 1). Eighteen species have most likely come from Australia, where they are native. Eight are global tramp species, commonly transported by human activities, and two species are of African origin. Nineteen of the currently established exotic species are recorded for the first time in New Zealand as occurring outside their native range. This may result in difficulty in obtaining species-specific biological knowledge and assessing their likelihood of becoming successful invaders. In addition to the work by Bolton (2003), Phil Ward and colleagues at UC Davis have started to resolve the phylogenetic relationships among subfamilies and genera of all ants using molecular data (Ward et al, 2005). -

Scope: Munis Entomology & Zoology Publishes a Wide Variety of Papers
Munis Entomology & Zoology Mun. Ent. Zool. https://www.munisentzool.org/ (January, 2021) 457 ISSN 1306-3022 © MRG ___________________________________________________________ EXPLORING THE ACULEATA HYMENOPTERA OF BANGLADESH BY DNA BARCODING OF MALAISE TRAP COLLECTION Santosh Mazumdar*, Paul D. N. Hebert** and Badrul Amin Bhuiya*** * Department of Zoology, University of Chittagong, BANGLADESH. E-mail: [email protected]; ORCID ID: 0000-0001-6403-577X ** Centre for Biodiversity Genomics, University of Guelph, 50 Stone Road East, Guelph, Ontario, CANADA. ORCID ID: 0000-0002-3081-6700 *** Biodiversity Research for Environment & Ecosystem Protection (BREEP), Chattogram- 4325, BANGLADESH. [Mazumdar, S., Hebert, P. D. N. & Bhuiya, B. A. 2021. Exploring the Aculeata Hymenoptera of Bangladesh by DNA barcoding of Malaise trap collection. Munis Entomology & Zoology, 16 (1): 457-464] ABSTRACT: Aculeata hymenopterans play a crucial role in ecology and economics. Diversity analysis of 901 Aculeata wasps at Chittagong University Campus a site in Bangladesh was performed by sequencing DNA barcodes (658 bp sequence from the 5′-end of cytochromeoxidase I). Specimens were collected by a Malaise trap from April 2014 to March 2015. The results revealed 22 species and 42 genera from 20 families in three superfamilies namely Apoidea, Chrysidoidea and Vespoidea. Among them 15 species, 22 genera, two subfamilies (Dolichoderinae and Ponerinae), and one family named Dryinidae are the first county records in Bangladesh. All the specimen records, with the Barcode Index Numbers (BINs) are available on the Barcode of Life Data System (BOLD). KEY WORDS: Aculeata Hymenoptera, Malaise trap, DNA barcode, Bangladesh Aculeata hymenopterans or stinging wasps (Hymenoptera: Aculeata) such as bees, ants, and many wasps play vital ecological roles as predators and pollinators and some have medicinal value as well (Zimmermann & Vilhelmsen, 2016). -

Hybridization in Ants
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2020 Hybridization in Ants Ian Butler Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons HYBRIDIZATION IN ANTS A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Ian Butler June 2020 © Copyright by Ian Butler 2020 HYBRIDIZATION IN ANTS Ian Butler, Ph.D. The Rockefeller University 2020 Interspecific hybridization is a relatively common occurrence within all animal groups. Two main factors make hybridization act differently in ants than in other species: eusociality and haplodiploidy. These factors serve to reduce the costs of interspecific hybridization in ants while simultaneously allowing them to take advantage of certain benefits. Eusociality may mitigate the effects of hybridization by allowing hybrids to be shunted into the worker caste, potentially reducing the effects of hybrid sterility. In haplodiploid species, males do not have a father. They instead develop from unfertilized eggs as haploid clones of their mother. This means that interspecifically mated queens do not completely sacrifice reproductive potential even if all hybrids are sterile because they can still produce fertile males. These factors in turn suggest that hybridization should be more common among the social Hymenoptera than other animal groups. Nevertheless, current data suggest that ants hybridize at rates similar to other animal groups, although these data are limited. Furthermore, there is a large amount of overlap between cases of interspecific hybridization and cases of genetic caste determination. A majority of the cases in ants where caste is determined primarily by genotype are associated with hybridization. -

Psyche [March
REVISION OF THE ANT TRIBE CARDIOCONDYLINI (HYMENOPTERA, FORMICIDAE) I. The Genus Prosopidris Wheeler BY JONATHAN REISKIND Biological Laboratories,, Harvard University This is the first of a series of papers revising the tribe Cardio- condylini, a group of small myrmicine ants (up to 3.o mm in length), characterized by the possession of a pedunculate: petiole, a wide postpetiolar dorsum, propodeal spines, and a general lack of pilosity. The tribe currently embraces the genera Cardiocondyla Emery (virtually cosmopolitan, mainly Old World), Xenometra Emery (West Indian) and Prosopidris (Papuasian). Xenometra includes a single poorly known species which is apparently parasitic on Cardio- condyla emeryi (Emery, I917). Cardiocondyla and Prosopidris are free living, and form small inconspicuous colonies: usually in the soil or in rotting logs. In this contribution Wheeler's subgenus Cardiocondyla (Prosopi- dris) is elevated to full generic status. The worker and female, types of its single previously known species, P. sima Wheeler (Philippines), are redescribed and a second species, P. PaDuana (New Guinea), is newly described. The papuana material includes the first known male of the genus, along with females and workers. T'he male is highly ergatoid, like those of some Cardiocondyla species. Measurements and Indices: In order to characterize properly cardio- condyline ants the following measurements and indices (with their abbreviations) will be used in this and succeeding papers: Head Length (HL) Maximum length of head in frontal view from clypeal apex to posterior border of occiput. Head Width (HW)Width of head measured in frontal view immediately behind the eyes. Scape Length (SL) Maximum measurable length of scape, not including its articular condyle. -
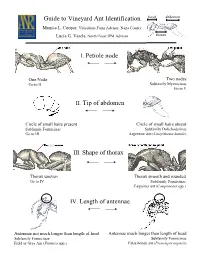
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

A Check List of the Ant Genus Crematogaster in Asia (Hymenoptera: Formicidae)
Bull. Inst. Trop. Agr., Kyushu Univ. 32: 43-83, 2009 43 A check list of the ant genus Crematogaster in Asia (Hymenoptera: Formicidae) Shingo HOSOISHI 1) and Kazuo OGATA1) Abstract A check list of the Asian species of the ant genus Crematogater is presented. The list covers the species-group names of the genus in Asia including the biogeographical areas of the eastern part of the Palearctic Region, the Oriental Region, and the western part of the Indo-Australian Region. A total of 206 names, comprising 145 species and 61 subspecies, is recognized. The list also provides information on the distribution. Introduction The ant genus Crematogaster was established by Lund in 1831, with the type-species, Formica scu- tellaris, which was subsequently designated by Bingham in 1903. The genus is one of mega-taxa of ants including 989 described names of species and subspecies from the world, in which there are 780 valid, 85 junior and 124 unavailable names according to the latest check list (Bolton et al., 2006). The genus is unique in having a characteristic connection of the postpetiol to the dorsal surface of the gaster, and easy to distinguish from other genera of the subfamily Myrmicinae. In spite of the dis- tinctness of the genus, the species level taxonomy is quite incomplete, and thus the exact figure of the taxa is still not clear. In Asia, biogeographical information of taxa is increasingly needed for studies of biodiversity, in particular, the species inventory of a local area. The term Asia is not biogeographical unit but a compos- ite of the eastern part of the Palearctic Region, the Oriental Region, and the western part of the Indo- Australian Region.