Psyche [March
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ORIGINAL ARTICLE a Novel Intracellular Mutualistic Bacterium in the Invasive Ant Cardiocondyla Obscurior
The ISME Journal (2016) 10, 376–388 © 2016 International Society for Microbial Ecology All rights reserved 1751-7362/16 www.nature.com/ismej ORIGINAL ARTICLE A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior Antonia Klein1,8, Lukas Schrader1,8, Rosario Gil2, Alejandro Manzano-Marín2, Laura Flórez3, David Wheeler5, John H Werren6, Amparo Latorre2,7, Jürgen Heinze1, Martin Kaltenpoth3,4, Andrés Moya2 and Jan Oettler1 1Institut für Zoologie, Universität Regensburg, Regensburg, Germany; 2Institut Canvanilles de Biodiversitat i Biologia Evolutiva (ICBiBE), Parc Cientific de la Universitat de Valencia, Paterna (Valencia), Spain; 3Max Planck Institute for Chemical Ecology, Jena, Germany; 4Johannes Gutenberg University Mainz, Institute for Zoology, Department for Evolutionary Ecology, Mainz, Germany; 5Institute of Fundamental Sciences, Massey University, Palmerston North, New Zealand; 6Department of Biology, University Rochester, Rochester, NY, USA and 7Área de Genómica y Salud de la Fundación para el Fomento de la Investigación Sanitaria y Biomédica de la Comunitat Valenciana (FISABIO)–Salud Pública, Valencia, Spain The evolution of eukaryotic organisms is often strongly influenced by microbial symbionts that confer novel traits to their hosts. Here we describe the intracellular Enterobacteriaceae symbiont of theinvasiveantCardiocondyla obscurior, ‘Candidatus Westeberhardia cardiocondylae’. Upon metamorphosis, Westeberhardia is found in gut-associated bacteriomes that deteriorate following eclosion. Only queens maintain Westeberhardia in the ovarian nurse cells from where the symbionts are transmitted to late-stage oocytes during nurse cell depletion. Functional analyses of the streamlined genome of Westeberhardia (533 kb, 23.41% GC content) indicate that neither vitamins nor essential amino acids are provided for the host. However, the genome encodes for an almost complete shikimate pathway leading to 4-hydroxyphenylpyruvate, which could be converted into tyrosine by the host. -

Hybridization in Ants
Rockefeller University Digital Commons @ RU Student Theses and Dissertations 2020 Hybridization in Ants Ian Butler Follow this and additional works at: https://digitalcommons.rockefeller.edu/ student_theses_and_dissertations Part of the Life Sciences Commons HYBRIDIZATION IN ANTS A Thesis Presented to the Faculty of The Rockefeller University in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy by Ian Butler June 2020 © Copyright by Ian Butler 2020 HYBRIDIZATION IN ANTS Ian Butler, Ph.D. The Rockefeller University 2020 Interspecific hybridization is a relatively common occurrence within all animal groups. Two main factors make hybridization act differently in ants than in other species: eusociality and haplodiploidy. These factors serve to reduce the costs of interspecific hybridization in ants while simultaneously allowing them to take advantage of certain benefits. Eusociality may mitigate the effects of hybridization by allowing hybrids to be shunted into the worker caste, potentially reducing the effects of hybrid sterility. In haplodiploid species, males do not have a father. They instead develop from unfertilized eggs as haploid clones of their mother. This means that interspecifically mated queens do not completely sacrifice reproductive potential even if all hybrids are sterile because they can still produce fertile males. These factors in turn suggest that hybridization should be more common among the social Hymenoptera than other animal groups. Nevertheless, current data suggest that ants hybridize at rates similar to other animal groups, although these data are limited. Furthermore, there is a large amount of overlap between cases of interspecific hybridization and cases of genetic caste determination. A majority of the cases in ants where caste is determined primarily by genotype are associated with hybridization. -
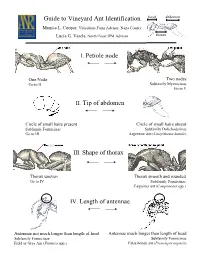
I. Petiole Node II. Tip of Abdomen IV. Length of Antennae Guide to Vineyard Ant Identification III. Shape of Thorax
Guide to Vineyard Ant Identification head abdomen Monica L. Cooper, Viticulture Farm Advisor, Napa County Lucia G. Varela, North Coast IPM Advisor thorax I. Petiole node One Node Two nodes Go to II Subfamily Myrmicinae Go to V II. Tip of abdomen Circle of small hairs present Circle of small hairs absent Subfamily Formicinae Subfamily Dolichoderinae Go to III Argentine Ant (Linepithema humile) III. Shape of thorax Thorax uneven Thorax smooth and rounded Go to IV Subfamily Formicinae Carpenter ant (Camponotus spp.) IV. Length of antennae Antennae not much longer than length of head Antennae much longer than length of head Subfamily Formicinae Subfamily Formicinae Field or Gray Ant (Formica spp.) False honey ant (Prenolepis imparis) head abdomen Petiole with two nodes Subfamily Myrmicinae (V-VIII) thorax V. Dorsal side of Thorax & Antennae One pair of spines on thorax No spines on thorax 12 segmented antennae 10 segmented antennae Go to VI Solenopsis molesta and Solenopsis xyloni VI. Underside of head No brush of bristles Brush of long bristles Go to VII Harvester ants (Pogonomyrmex californicus and P. brevispinosis) VII. Head and Thorax With hairs Without hairs Go to VIII Cardiocondyla mauritanica VIII. Head and Thorax With many parallel furrows Without parallel furrows Profile of thorax rounded Profile of thorax not evenly rounded Pavement ant (Tetramorium “species E”) Pheidole californica Argentine Ant (Linepithema humile), subfamily Dolichoderinae Exotic species 3-4 mm in length Deep brown to light black Move rapidly in distinct trails Feed on honeydew Shallow nests (2 inches from soil surface) Alex Wild Does not bite or sting Carpenter Ant (Camponotus spp.), subfamily Formicinae Large ant: >6 mm in length Dark color with smooth, rounded thorax Workers most active at dusk and night One of most abundant and widespread genera worldwide Generalist scavengers and predators: feed on dead and living insects, nectar, fruit juices and Jack K. -

Recent Human History Governs Global Ant Invasion Dynamics
ARTICLES PUBLISHED: 22 JUNE 2017 | VOLUME: 1 | ARTICLE NUMBER: 0184 Recent human history governs global ant invasion dynamics Cleo Bertelsmeier1*, Sébastien Ollier2, Andrew Liebhold3 and Laurent Keller1* Human trade and travel are breaking down biogeographic barriers, resulting in shifts in the geographical distribution of organ- isms, yet it remains largely unknown whether different alien species generally follow similar spatiotemporal colonization patterns and how such patterns are driven by trends in global trade. Here, we analyse the global distribution of 241 alien ant species and show that these species comprise four distinct groups that inherently differ in their worldwide distribution from that of native species. The global spread of these four distinct species groups has been greatly, but differentially, influenced by major events in recent human history, in particular historical waves of globalization (approximately 1850–1914 and 1960 to present), world wars and global recessions. Species in these four groups also differ in six important morphological and life- history traits and their degree of invasiveness. Combining spatiotemporal distribution data with life-history trait information provides valuable insight into the processes driving biological invasions and facilitates identification of species most likely to become invasive in the future. hallmark of the Anthropocene is range expansion by alien been introduced outside their native range). For each species, we species around the world1, facilitated by the construction of recorded the number of countries where it had established (spatial transport networks and the globalization of trade and labour richness) and estimated spatial diversity taking into account pair- A 2 16 markets since the beginning of the Industrial Revolution . -

Nest Density of Aneuretus Simoni Emery (Sri Lankan Relict Ant) and Stereomyrmex Horni Emery in Three Forest Regions in Western and Southern Sri Lanka
ASIAN MYRMECOLOGY Volume 6, 83–90, 2014 ISSN 1985-1944 © R. K. S. DIA S AN D H. P. G. R. C. RU ch I R ANI Nest density of Aneuretus simoni Emery (Sri Lankan Relict Ant) and Stereomyrmex horni Emery in three forest regions in western and southern Sri Lanka R. K. S. DIA S AN D H. P. G. R. C. RU ch I R ANI Department of Zoology, University of Kelaniya, Sri Lanka Corresponding author’s e-mail: [email protected] ABSTRACT. Aneuretus simoni Emery and Stereomyrmex horni Emery are two endemic and rare ant species in Sri Lanka. The presence or absence and nest density of these species was surveyed to find out if they inhabit locations outside the previously recorded habitats, and to verify their conservation status. Colonies were surveyed by thorough inspection, breaking decaying pieces of wood and the removal of leaf litter in a forest in Western Province (Kalugala Proposed Forest Reserve – KPFR) and two forests in Southern Province (Kuluna Kanda Proposed Forest Reserve – KKPFR and Wilpita “Aranya Kele” in Oliyagan Forest Reserve – WFR). Aneuretus simoni nests were recorded in 7.5% of quadrats, with a nest density of 0.30 nests m−2 at the site of KPFR, whereas this species was more common at KKPFR , occurring in 23.3% of samples with a nest density of 0.93 nests m−2. At WFR site, A. simoni nests were found in 6.7% of samples at a nest density of 0.27 nests m−2. Stereomyrmex horni Emery was found in 15% of quadrats at 0.6 nests m−2 at KPFR and 3% of quadrats at 0.13 nests m−2 at WFR. -

Phylogeny and Biogeography of a Hyperdiverse Ant Clade (Hymenoptera: Formicidae)
UC Davis UC Davis Previously Published Works Title The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae) Permalink https://escholarship.org/uc/item/2tc8r8w8 Journal Systematic Entomology, 40(1) ISSN 0307-6970 Authors Ward, PS Brady, SG Fisher, BL et al. Publication Date 2015 DOI 10.1111/syen.12090 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Systematic Entomology (2015), 40, 61–81 DOI: 10.1111/syen.12090 The evolution of myrmicine ants: phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae) PHILIP S. WARD1, SEÁN G. BRADY2, BRIAN L. FISHER3 andTED R. SCHULTZ2 1Department of Entomology and Nematology, University of California, Davis, CA, U.S.A., 2Department of Entomology, National Museum of Natural History, Smithsonian Institution, Washington, DC, U.S.A. and 3Department of Entomology, California Academy of Sciences, San Francisco, CA, U.S.A. Abstract. This study investigates the evolutionary history of a hyperdiverse clade, the ant subfamily Myrmicinae (Hymenoptera: Formicidae), based on analyses of a data matrix comprising 251 species and 11 nuclear gene fragments. Under both maximum likelihood and Bayesian methods of inference, we recover a robust phylogeny that reveals six major clades of Myrmicinae, here treated as newly defined tribes and occur- ring as a pectinate series: Myrmicini, Pogonomyrmecini trib.n., Stenammini, Solenop- sidini, Attini and Crematogastrini. Because we condense the former 25 myrmicine tribes into a new six-tribe scheme, membership in some tribes is now notably different, espe- cially regarding Attini. We demonstrate that the monotypic genus Ankylomyrma is nei- ther in the Myrmicinae nor even a member of the more inclusive formicoid clade – rather it is a poneroid ant, sister to the genus Tatuidris (Agroecomyrmecinae). -

First Records of Cardiocondyla Ants from Myanmar
ASIAN MYRMECOLOGY — Volume 12, e012005, 2020 ISSN 1985-1944 | eISSN: 2462-2362 DOI: 10.20362/am.012005 First records of Cardiocondyla ants from Myanmar Jürgen Heinze1*, Andreas Trindl1, Bernhard Seifert2, Khin Ma Ma3, and Win Maung4 1LS Zoologie / Evolutionsbiologie, Universität Regensburg, 93040 Regensburg, Germany 2Department of Entomology, Senckenberg Museum für Naturkunde, 02826 Görlitz, Germany 3Bago University, Bago, Myanmar 4Myanmar Environment Institute, Yangon, Myanmar *Corresponding author: [email protected] ABSTRACT. Numerous species of the diverse ant genus Cardiocondyla have been reported from countries in Southeast Asia, but as yet nothing has been known about their presence in Myanmar. A short, preliminary survey documents the occurrence of at least five species of Cardiocondyla in anthropogenically disturbed areas, such as gardens and parks. The species most commonly sampled has been determined as C. tjibodana, other specimens as C. parvinoda, C. obscurior, C. itsukii and one or two taxa of the unresolved C. kagutsuchi complex. Further studies, in particular in more natural habitats, will certainly reveal the presence of additional species of this highly interesting genus. Keywords Cardiocondyla, biodiversity, invasive ants, Myanmar Citation Jürgen Heinze et al. (2020) First records of Cardiocondyla ants from Myanmar. Asian Myrmecology 12: e012005 Copyright This article is distributed under a Creative Commons Attribution License CCBY4.0 Communicating Wendy Wang Editor INTRODUCTION Cardiocondyla species have wingless, “ergatoid” males, which either co-occur with or have com- The myrmicine genus Cardiocondyla is of con- pletely replaced winged disperser males. In some siderable interest as it shows a striking diversity species, these wingless males are fierce fight- of life histories, reproductive strategies of its ers, which engage in fatal combat for access to males, and ecological preferences (Heinze 2017). -

Rabeling, C. 2020. Social Parasitism. In
S Social Parasitism life cycle, such as colony founding, but are other- wise able to live independently without the host’s Christian Rabeling help. Alternatively, social parasites can be obli- School of Life Sciences, Arizona State University, gately dependent on their hosts’ social behavior. Tempe, AZ, USA Among the Hymenoptera, social parasitism is commonly found in ants, social bees, and social wasps [25] (Figs. 1–4). However, it is not always In the broadest sense, social parasitism is a form of easy to recognize a true social parasite because brood parasitism, where the social parasite insect societies are readily exploited by scaven- depends on the social behavior of the host for gers, parasites, and predators, and consequently, survival and reproduction. Brood parasitism is nests of social insects are riddled with “guests” known from a variety of vertebrate species, such [10, 25]. Although highly intriguing and directly as mammals, birds, and fishes, where the host’s relevant to understanding the rich biology of brood care behavior is exploited by the parasite. social parasites, this overview primarily focuses Brood parasitism has been studied in great detail on interspecific social parasitism among eusocial in some bird species, including cuckoos, cow Hymenoptera. Other symbiotic interactions birds, and honeyguides, where parasites lay their between social insects and other organisms, such eggs into the nests of the host, deceiving the host as intraspecific parasitism (which is probably a into providing parental care for their offspring. widespread but often overlooked form of social In social insects, social parasites also exploit parasitism occurring in polygynous colonies of a the brood care behavior of their hosts and can single species), myrmecophily, interactions therefore be regarded as brood parasites. -

Worldwide Spread of the Moorish Sneaking Ant, Cardiocondyla Mauritanica (Hymenoptera: Formicidae) by James K
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Portal de Periódicos Eletrônicos da Universidade Estadual... 985 Worldwide Spread of the Moorish sneaking Ant, Cardiocondyla mauritanica (Hymenoptera: Formicidae) by James K. Wetterer ABSTRACT Cardiocondyla spp. are small, inconspicuous ants, native to the Old World. Until recently, Cardiocondyla mauritanica Forel, 1890 was a little known species recorded almost exclusively from the semi-arid subtropics of North Africa, the Middle East, and neighboring islands. In contrast, Cardiocondyla nuda Mayr, 1866 was considered a cosmopolitan tramp species, spread broadly around the world through human commerce. A recent taxonomic reanalysis by B. Seifert, however, found genuine C. nuda restricted to Australia, New Guinea, and Western Oceania, and that published records of ‘C. nuda’ from outside this region were based on misidentifications of other species, notablyC. mauritanica. In addition, Cardiocondyla ectopia, known from North America, was found to be a junior synonym of C. mauritanica. Here, I examine the worldwide spread of C. mauritanica. I compiled published and unpublished C. mauritanica specimen records from >250 sites, documenting the earliest known records for 47 geographic areas (countries, island groups, major islands, and US states), including sev- eral for which I found no previously published records: Barbados, Bonaire, Curaçao, Grenada, Saba, and Saudi Arabia. Cardiocondyla mauritanica is found primarily in semi-arid and urban environments. Cardiocondyla mauritanica shows an apparently continuous distribution and geographic variation in morphology from northwest Africa to India suggesting that C. mauritanica is native throughout this subtropical expanse. Old World records of C. mauritanica far from this range come from Ascension, Zimbabwe, and several Indo-Pacific islands. -

Crystalline Deposits Reveal Caste Identity of Late Embryos and Larvae of the Ant
bioRxiv preprint doi: https://doi.org/10.1101/2021.08.13.456267; this version posted August 14, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 1 Crystalline deposits reveal caste identity of late embryos and larvae of the ant 2 Cardiocondyla obscurior 3 4 Tobias Wallner1 5 Eva Schultner1 6 Jan Oettler1* 7 8 1Zoology/Evolutionary Biology, University of Regensburg, Universitätsstrasse 31, 9 93053 Regensburg, Germany 10 11 *corresponding author 12 13 ORCID: 14 Tobias Wallner: 0000-0001-9135-6456 15 Eva Schultner: 0000-0002-5069-9732 16 Jan Oettler: 0000-0002-8539-6029 17 18 Keywords: 19 Caste; social insects, ant larvae; urate; ovarian development; eco-evo-devo bioRxiv preprint doi: https://doi.org/10.1101/2021.08.13.456267; this version posted August 14, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. 20 Abstract 21 Social insects are interesting models for the study of anticipatory developmental 22 plasticity because of the striking differentiation into reproductive queens and 23 functionally sterile workers. A few ant genera, including Cardiocondyla, represent the 24 pinnacle of social evolution in the Hymenoptera, where workers have completely lost 25 their reproductive organs, minimizing reproductive conflicts between queens and 26 workers. -

Hymenoptera: Formicidae
ISSN 1997-3500 Myrmecological News myrmecologicalnews.org Myrmecol. News 29: 163-166 doi: 10.25849/myrmecol.news_029:163 18 December 2019 Original Article First record of the formicine genus Overbeckia (Hymeno ptera: Formicidae) from Australia Brian E. Heterick Abstract A collection of ants deeded to the Western Australian Museum by visiting amateur Raul Jordan included representatives of 10 subfamilies, 65 genera and 270 species. The ants were collected in 2002 in the Northern Territory, Queensland and Western Australia. Among the specimens were three workers of Overbeckia collected in Mossman, Queensland. This is an extremely rare arboreal genus known previously from only two collections in Singapore and the Philippines. The sole known species is Overbeckia subclavata Viehmeyer, 1916. The Australian material is hairier but otherwise agrees exactly with syntype workers illustrated on AntWeb. The implications of this find are discussed. The alternatives include (i) a new species of Overbeckia possibly endemic to Australia, (ii) the extension of the known range of Over- beckia subclavata and (iii) the introduction to Australia of a hairy morph of O. subclavata by human agency. At present, insufficient material is available to enable this author to choose between these alternatives. The Jordan Collection also includes the first Australian records of Solenopsis papuana Emery, 1900 and Cardiocondyla minutior Forel, 1899. These three new records are of considerable significance, and the value of amateur collections and the importance of good taxonomy are discussed in this paper. Key words: Overbeckia, Formicinae, Australia, Raul Jordan Collection, relevance of museum collections. Received 7 October 2019; revision received 19 November 2019; accepted 26 November 2019 Subject Editor: Andrew V. -

Biodiversity Conservation in a Fragmented Landscape: Arthropod Assemblages in Smaller Corridors Within a Production Landscape
Biodiversity conservation in a fragmented landscape: arthropod assemblages in smaller corridors within a production landscape by Julia van Schalkwyk Thesis presented in partial fulfilment of the requirements for the degree of Master of Science (Conservation Ecology) in the Faculty of AgriSciences at Stellenbosch University Supervisors: Dr. James S. Pryke and Prof. Michael J. Samways Department of Conservation Ecology and Entomology Faculty of AgriSciences Stellenbosch University March 2015 Stellenbosch University https://scholar.sun.ac.za Declaration By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights, and that I have not previously in its entirety, or in part, submitted it for obtaining any qualification. October 2014 Copyright © 2015 Stellenbosch University All rights reserved II Stellenbosch University https://scholar.sun.ac.za All rights reserved Overall summary Habitat loss and fragmentation are major threats to global biodiversity. A cornerstone of traditional conservation involves setting aside land as formally protected areas (PAs). However, for effective biological conservation in the long term there needs to be connectivity between these PAs. When possible, improved connectivity can be achieved using natural corridors at a landscape scale. Even better is to establish a network of corridors and nodes in the form of ecological networks (ENs). ENs are currently being employed by commercial forestry companies in South Africa. While larger corridors and nodes are considered optimum, factors other than design, such as management and environmental heterogeneity, have also been found to be important for species maintenance.