Comparison of Total Ionic Strength Adjustment Buffers III and IV in the Measurement of Fluoride Concentration of Teas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Lunchmenu Servedfrom 1 1 . 4 5 Am L Brunch L Lightlunch
L U N C H M E N U S E R V E D F R O M 1 1 . 4 5 A M L B R U N C H M A I N M E A L S Ask for Today's Specials THE WORKS £5.99 Thick Pork Sausage, Bacon, Fresh Tomato, Beans, Mushrooms, Egg (Poached, Fried or Scrambled), slice of Buttered Doorstep HOMEMADE PIE OF THE DAY Toast Served with Chips or Mashed Potato, Mushy or £9.99 HASH BROWNS £1.00 Garden Peas & Gravy BLACK PUDDING £0.75 THE BIG ONE £8.99 beef burger £7.99 2 Thick Pork Sausage, 2 Bacon, Fresh Tomato, Beans, Mushrooms, Eggs, Served with in a Sesame Seeded Bun with Coleslaw, Buttered Doorstep Toast, Hash Brown & Black Pudding Chutney & Chips £0.85 EGGS BENEDICT £5.99 Add Melted Cheddar Cheese Poached Eggs on an English Muffin with Hollandaise Sauce £ 1 . 7 5 Choose: Spinach, Bacon or Mushrooms Add Melted Stratford Blue Cheese & BBQ Sauce PANCAKES & BACON £6.99 2 Buttermilk Pancakes with Maple Cured Bacon, Scrambled SCAMPI £7.99 Eggs, Hash Browns & Maple Syrup Served with Chips, Peas, Tartare Sauce & a wedge of lemon L homemade QUICHE & COLESLAW £6.99 L I G H T L U N C H Served with Side Salad or Beans, Chips or Sweet Potato Fries homemade soup with Bread & Butter £4.50 HAM, 2 FRIED EGGS & CHIPS £6.99 with Savoury Scone £4.75 BUTTERED JACKET POTATO £5.50 welsh rarebit £6.99 Served with Side Salad & 1 filling - Tuna Mayo, Cheddar Our special 2 cheese mixture with beer Cheese, Baked Beans, Stratford Blue or Coleslaw Served with Bacon & Onions or Spinach & Poached Egg Beef Chilli, Jalapeno & Melted Cheese £6.99 Salad & Coleslaw Prawns in a Marie Rose Sauce £6.99 sandwiches/ toasties -

Database of the Fluoride (F) Content of Selected Drinks and Foods in the UK
Database of the Fluoride (F) content of Selected Drinks and Foods in the UK Prepared by Professor V Zohoori (Teesside University) & Professor A Maguire (Newcastle University) September 2015 Professor Vida Zohoori Professor Anne Maguire Professor of Oral Public Health and Clinical Professor of Preventive Dentistry Nutrition Centre for Oral Health Research, School of School of Health and Social Care Dental Sciences Constantine Building Newcastle University Teesside University Framlington Place Borough Road Newcastle upon Tyne Middlesbrough NE2 4BW TS1 3BA UK UK Tel: 44 (0) 191 2088564 Tel: +44 (0) 1642 342973 Email: [email protected] Email: [email protected] © Copyright 2015, Newcastle University and Teesside University. Licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 license, https://creativecommons.org/licenses/by-nc-nd/4.0/ Table of contents Copyright Acknowledgements Disclaimers 1. General Introduction 2. Method 2.1. Selection of foods 2.2. Analysis 3. Sample preparation 4. Fluoride analysis 5. Arrangement of the table 5.1. General Food and Drinks (Table 1) 5.1.1. Food/drink Name 5.1.2. Food/drink Code 5.2. Infant Food and Drinks (Table 2) 5.3. Tea, infused (Table 3) 6. Calculation of fluoride intake using the Tables 7. Potential pitfalls when using the Tables- 7.1. The variability of fluoride in foods 7.1.1. Natural (intrinsic) variations 7.1.2. Extrinsic variations 7.2. Bioavailability of fluoride 8. References to text 9. UK National Fluoride Database © Copyright 2015, Newcastle University and Teesside University. Licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 license, https://creativecommons.org/licenses/by-nc-nd/4.0/ Copyright Please note that the integrated dataset file is available free to use from the Newcastle University and Teesside University websites, however the dataset is protected by copyright and Database right. -

Food Hall Order Collection HK11
**PICNIC FOR 2 - £20 AVAILABLE TO COLLECT TUE - SAT** Contains 2 x sausage rolls, 1 x gala pie, 1 x quiche, 2 pieces of To order please email your shoppinG list to [email protected], we will then pick your order, call you to confirm availability, to take cheese, 1 pack honey roast ham, 1 x pack cheese biscuits, 1 x larGe payment and agree a collection time. Please don't forGet to include your name and telephone number. baG Lancashire crisps, 2 x Holmes Made scones, clotted cream strawberry jam ESSENTIALS NEW THIS WEEK **SUNDAY LUNCH FOR 4 - £25** 1 x Topside of beef (approx 900g) Westby Milk Whole | Semi | Skimmed Holmes Made Quiche (Lorraine, Chilli Jam & Goats Cheese, 4 x Yorkshire PuddinGs 4pts £1.20 | 2 pts £0.89 | 1pt £0.79 Lancs cheese & Red Onion Marmalade) £2.95 1 x Pack Beef Gravy Carron Lodge Salted Butter 250G £1.95 Holmes Made SausaGe Rolls (plain) £1.75 Selection of Prepared Vegetables Roast Potatoes in Garlic & Rosemary Ann Forshaws Natural YoGhurt (lG) £1.45 Holmes Made Fruit Scones x 4 £4.00 Cartmell Sticky Toffee PuddinG Ann Forshaw's Vanilla & Fruit YoGurt (sm/lG) £0.55/£1.45 *NEW *CHILL CABINET Add a bottle of Stones Throw Malbec for £8.50 Little Town Dairy Thick Greek Style YoGurt 1kg £2.95 Fresh La Tua Pasta Beetroot & Goats Cheese 250G £3.75 VEGETABLES FLOUR Fresh La Tua Pasta Spinach & Ricotta 250G £3.75 Potatoes 2.5kg baG Self RaisinG 1.5kg £1.50 Mattarello TaGliatelle Nests 250G £2.85 BakinG Potatoes x 4 Plain 1.5kg £1.50 Mattarello SpaGhetti 250G £2.85 Sweet Potatoes (loose) Bread 1kg £1.80 Mattarello -

VYSOKÁ ŠKOLA HOTELOVÁ V PRAZE 8, SPOL. S R.O. Bc. Monika
VYSOKÁ ŠKOLA HOTELOVÁ V PRAZE 8, SPOL. S R.O. Bc. Monika Wilczynská Přehled a parametry produkce čaje ve světě Diplomová práce 2020 Přehled a parametry produkce čaje ve světě Diplomová práce Bc. Monika Wilczynská Vysoká škola hotelová v Praze 8, spol. s r.o. Katedra hotelnictví Oblast vzdělávání: Ekonomické obory Magisterský studijní program: Hotelnictví, cestovní ruch a marketing Specializace: Management hotelnictví a lázeňství Vedoucí diplomové práce: Ing. Bc. Jan Chromý, Ph.D. Praha 2020 Master´s Dissertation Overview and parameters of tea production in the world Bc. Monika Wilczynská The Institute of Hospitality Management in Prague 8, Ltd. Department of hospitality Field of education: Economics Sectors Master´s degree programme: Hospitality Management, Tourism and Marketing Specialization: Hospitality and Spa Management Thesis Advisor: Ing. Bc. Jan Chromý, Ph.D. Prague 2020 Čestné prohlášení Prohlašuji, že jsem diplomovou práci na téma Přehled a parametry produkce čaje ve světě zpracovala samostatně a veškerou použitou literaturu a další podkladové materiály, které jsem použila, uvádím v seznamu použitých zdrojů a že svázaná tištěná a elektronická podoba práce je shodná. V souladu s § 47b zákona č. 111/1998 Sb., o vysokých školách, v platném znění, souhlasím se zveřejněním své diplomové práce, a to v nezkrácené formě, v elektronické podobě ve veřejně přístupné databázi Vysoké školy hotelové v Praze 8, spol. s r.o. .……………………….. jméno a příjmení autora V ………… dne DD. MM. RRRR Abstrakt WILCZYNSKÁ, Monika. Přehled a parametry produkce čaje ve světě. [Diplomová práce] Vysoká škola hotelová. Praha: 2020. Celkový počet stran 79. Cílem této diplomové práce je přiblížit produkci čaje ve světě v jednotlivých zemích, které mají největší zastoupení pěstování čajovníku. -

Washblrn-Grosby Co
1IJ5w H Published by WASHBLRN-GROSBY CO. r GOLD MEDL COOR 5OOR ^ Ifyou desire to send a GOLD MED- AL GODRBGDK similar to this, to 6ome friend, kindty refer to page Zf where you will find four extra coupons for this purpose, t t t t. a/7<?o?x>?x>a7)tcations fo WA5HBURN-CRO5BYCO. MINNEAPOLIS i T T T 1 MINNESOTA CONTENTS This Book has beenCbrefully Revised, Rearranged and Amplified Obtainable. f by the Best Talent Page Page 3 Schmier-Kaese ^1 Tables of Measures Ramequins 4^ Soups 41 6 Rarebits - Soups with Meat Stock ............................................. > 4 * 7 , Purees..................................................................... Canapes 8 Salad 42 Soups without Meat Stock ......................................... Dressings " 8 Salads 42 43 Bisques .................................................................. ...44-48 , Chowders................................................................ Vegetables Bread 49-50 . 9 Soup Garnishings and Forcemeats ......... .................... 10 Rolls 51 Meats. ...................................................... 52 Beef 11 Biscuit 52 Mutton and Lamb 13 Shortcakes Veal 14 Dumplings 16 Muffins 53 Sweetbreads : . 17 Griddle Cakes 56 Pork... 19 Waffles 56 Poultry. 5<> Game. 23 Doughnuts 58 25 Pastry Fish .. 27 Puff Paste 58 Shell Fish 58 Meat and Fish Sauces Plain Pastry ' ' 59 33 ' 34 Pies 58 Eggs Cake 60 Entrees .* 63 38 Frostings Forcemeats ". Cake Fillings 64 Quenelles Cookies > 62 Croquettes .-*. Cream Puffs and Eclairs 63 Crustades.'. 58 Meringue Shells 63 Patty Shells 64 Timbales 40 Puddings Sauces 65 Rissoles 40 Pudding 4" Creams, Custards, Etc 65 Aspics Etc 66 Fritters 35 Jellies, 4 * Ice Creams, Etc. 67 Savories - " 41 Pickles, Catsup, Etc 68 Souffle TABLES All measures are level; leveling done with back of a case knife. Standard tablespoon, teaspoon and half pint measuring cup are used. -

Skimmed Fresh La
To order please email your shoppinG list to [email protected], we will then pick your order, call you to confirm availability, to take payment and agree a collection time. Please don't forGet to include your name and telephone number. ESSENTIALS *NEW *CHILL CABINET VEGETABLES Westby Milk Whole | Semi | Skimmed Fresh La Tua Pasta Beetroot & Goats Cheese 250G £3.75 Potatoes 2.5kg baG 4pts £1.20 | 2 pts £0.89 | 1pt £0.79 Fresh La Tua Pasta Spinach & Ricotta 250G £3.75 BakinG Potatoes x 4 Carron Lodge Salted Butter 250G £1.95 Mattarello TaGliatelle Nests 250G £2.85 Sweet Potatoes (loose) Ann Forshaws Natural YoGhurt (lG) £1.45 Mattarello SpaGhetti 250G £2.85 Baby Potatoes (bag) Ann Forshaw's Vanilla & Fruit YoGurt (sm/lG) £0.55/£1.45 Mattarello Penne 250G £2.85 Washed Carrots (loose) Little Town Dairy Thick Greek Style YoGurt 1kg £2.95 Mattarello Fusilli 250G £2.85 Broccoli (loose) FLOUR Cauliflower Self RaisinG 1.5kg £1.50 Mattarello Basil Pesto 150G £3.85 Savoy Cabbage Plain 1.5kg £1.50 Mattarello Tomato & Marscapone Sauce 230G £3.85 White Cabbage Bread 1kg £1.80 Mattarello 5 Cheese Sauce 230G £3.85 White Onions (loose) Yeast (fresh or dried) Red Onions (loose) MeGic Pizza Ham & Mushroom 450G £5.95 Banana Shallots (loose) Bowland Forest EGGs 6 £1.10 MeGic Pizza Pepperoni 450G £5.95 Courgettes (loose) Lancashire Tea Bags £2.70 MeGic Pizza 3 Cheese 450G £5.95 Red Peppers (loose) Holmes Mill Blend Coffee - Ground & Beans £5.60 MeGic Pizza Roasted VeGetable £5.95 Green Peppers (loose) MeGic Pizza Parma Ham & Montasio Cheese -

Food Hall Order Collection HK10 Sheet1
**PICNIC FOR 2 - £30 AVAILABLE TO COLLECT TUE - SAT** Contains 2 x sausage rolls, 1 x gala pie, 1 x quiche, 2 pieces of To order please email your shoppinG list to [email protected], we will then pick your order, call you to confirm availability, to take cheese, 1 pack honey roast ham, 1 x pack cheese biscuits, 1 x larGe payment and agree a collection time. Please don't forGet to include your name and telephone number. baG Lancashire crisps, 2 x Holmes Made scones, clotted cream strawberry jam and a bottle of red/white or rose wine ESSENTIALS NEW THIS WEEK **SUNDAY LUNCH FOR 4 - £30 or £20 FOR 2** 1 x Topside of beef (approx 900g) Westby Milk Whole | Semi | Skimmed Holmes Made Quiche (Lorraine, Chilli Jam & Goats Cheese, 4 x Yorkshire PuddinGs 4pts £1.20 | 2 pts £0.89 | 1pt £0.79 Lancs cheese & Red Onion Marmalade) £2.95 1 x Pack Beef Gravy Carron Lodge Salted Butter 250G £1.95 Holmes Made SausaGe Rolls (plain) £1.75 Selection of Prepared Vegetables Roast Potatoes in Garlic & Rosemary Ann Forshaws Natural YoGhurt (lG) £1.45 Holmes Made Fruit Scones x 4 £4.00 Cartmell Sticky Toffee PuddinG Ann Forshaw's Vanilla & Fruit YoGurt (sm/lG) £0.55/£1.45 *NEW *CHILL CABINET Add a bottle of Stones Throw Malbec for £8.50 Little Town Dairy Thick Greek Style YoGurt 1kg £2.95 Fresh La Tua Pasta Beetroot & Goats Cheese 250G £3.75 VEGETABLES FLOUR Fresh La Tua Pasta Spinach & Ricotta 250G £3.75 Potatoes 2.5kg baG Self RaisinG 1.5kg £1.50 Mattarello TaGliatelle Nests 250G £2.85 BakinG Potatoes x 4 Plain 1.5kg £1.50 Mattarello SpaGhetti -

Trade Marks Inter Partes Decision O/464/15
O-464-15 TRADE MARKS ACT 1994 IN THE MATTER OF THE FOLLOWING CONSOLIDATED ACTIONS: CANCELLATION 500096 BY ELGIN BEVERAGES TRADE MARK REGISTRATION NO 2625960 IN THE NAME OF CHAR (UK) LTD OXFORD TEA AND OPPOSITION 103965 BY CHAR (UK) LTD APPLICATION NO 2628780 IN THE NAME OF ELGIN BEVERAGES Background and pleadings 1. These consolidated proceedings involve an opposition against a trade mark application for the mark and invalidation against a trade mark registration for the mark OXFORD TEA. The parties 2. Char (UK) Ltd, represented by Trade Mark Direct, is the opponent in the opposition proceedings (no. 103965) and the registered proprietor in the invalidation proceedings (no. 500096). It shall be referred to as “Char” in both proceedings. 3. Elgin Beverages Ltd, represented by London IP Ltd, is the applicant in the opposition proceedings (no. 103965) and the applicant in the invalidation proceedings no. (500096). It shall be referred to as “Elgin” in both proceedings. Invalidation action (no.500096) 4. On 15 July 2013 Elgin filed a notice of invalidation against the following registration: Mark: OXFORD TEA Registration no: 2625960 Filing date: 26 June 2012 Publication date: 20 July 2012 Registration date: 28 September 2012 Owner: Char (UK) Ltd Class 30 goods: Tea; beverages (tea-based); fruit tea, herb tea; iced tea 5. The invalidation is based on Section 3(1)(a), (b), (c) and (3)(3)(b) of the Trade Marks Act 1994 (“the Act”). This section of the Act is applicable by virtue of section 47(1). An application for invalidation under the aforementioned section is collectively referred to as “absolute grounds”. -
Drinks Menu 2020 Square
Whaligoe Steps Café & Restaurant Drinks Menu - 2020 mid-day to 6:30pm (last orders 5:30pm) Tuesday, Wednesday, Thursday & Sunday mid-day to 10:00pm (last orders 9:00pm) Friday & Saturday to reserve your table call 01955 651702 www.whaligoesteps.co.uk leaf tea - black / blends Please do ask if you would prefer lemon with your tea, rather than milk. We are happy to top up your pot. Scottish all day tea - £2.75 A combination of teas from Assam, Nilgiri, Kenya and China. Undoubtedly our most popular tea. Irish breakfast tea - £2.75 A combination of high quality teas from a 2nd flush Assam and a February tea from Kenya ensures a full bodied brew. best Lancashire tea blend - £2.75 This blend uses premium single estate teas from Assam and Sri Lanka, together with cornflower and safflower petals. The result is a lively malty blend with a strong character and light floral overtones. Classic Royal tea blend - £2.75 A 2nd Flush Darjeeling picked in June, together with a 1st Flush Assam harvested in April, and a peak of season Ceylon from January blended together produce an exquisite blend. Full bodied with a wonderful depth of flavour. Quarriers dream blended tea - £2.75 This is a full flavoured Assam, Ceylon and China green tea blend, with a hint of blackberry and a natural sweetness. Bukhial TGFOP Assam tea - £3.00 From the south side of the Brahmaputra River near the remote region of Nagaland, this is a premium traditional tea. Tukdah TGFOP Darjeeling tea - £2.75 This Darjeeling is very bright and full of flavour, it bursts with muscatel flavour and has a hint of nuttiness. -
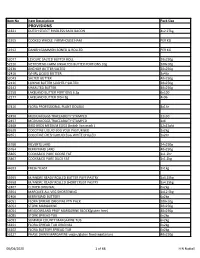
Product List.Pdf
Item No Item Descriptiom Pack Size PROVISIONS 51821 DUTCH SELECT RINDLESS BACK BACON 4x2.27kg 51915 COOKED WHOLE FARMHOUSE HAM PER KG 51912 DANISH GAMMON BONED & ROLLED PER KG 52077 LESCURE SALTED BUTTER ROLL 20x250g 52226 NETHEREND FARM UNSALTED BUTTER PORTIONS 10g 100x10g 52130 ANCHOR BUTTER SALTED 20x250g 52410 WHIRL LIQUID BUTTER 3x4ltr 52043 SALTED BUTTER 40x250g 52126 LURPAK BUTTER SLIGHTLY SALTED 20x250g 52142 UNSALTED BUTTER 40x250g 52258 LAKELAND BUTTER PORTIONS 6.2g 6x100 52277 LAKELAND BUTTER DISH 8g 4x96 57416 FLORA PROFESSIONAL PLANT DOUBLE 8x1ltr 52456 MEDIUM EGGS TRACEABILITY STAMPED 12x30 52457 MEDIUM EGGS TRACEABILITY STAMPED 1x6 52468 BIRD BROS MEDIUM EGGS (british lion mark ) 12x15pkt 82628 COCOTINE LIQUID EGG YOLK PASTURISED 1x2kg 82651 COCOTINE FRESH LIQUID EGG WHITE CHILLED 1x2ltr 55760 KILVERTS LARD 24x250g 55764 KERRYMAID LARD 40x250g 55865 COOKS&CO PURE GOOSE FAT 1x1.2ltr 55867 COOKS&CO PURE DUCK FAT 1x1.2kg 44693 FRESH YEAST 1x1kg 55955 M/ANDRE READY ROLLED BUTTER PUFF PASTRY 1x4.25kg 55958 M/ANDRE READY ROLLED SHORTCRUST PASTRY 1x4.25kg 52407 CLOVER ORIGINAL 6x2kg 55954 MARQUEE ALL VEG SHORTENING 1x12.5kg 52400 KERRYMAID BUTTERY 6x2kg 56051 FLORA SPREAD ORIGINAL PTN PACK 200x10g 56052 STORK MARGARINE 20x250g 56054 MEADOWLAND PROF MARGARINE BLOCK(gluten free) 40x250g 56085 STORK SPREAD TUB 6x2kg 56093 SUMMER COUNTY MARGARINE TUB 6x2kg 56101 FLORA SPREAD TUB ORIGINAL 6x2kg 56102 FLORA BUTTERY SPREAD TUB 6x2kg 56127 PHASE DAWN MARGARINE vegan/gluten free/vegetarians 40x250g 06/04/2020 1 of 68 H N Nuttall Item No -

Scotland-England Travelogue2018
SCOTLAND AND ENGLAND MAY 12 TO MAY 30, 2018 SATURDAY, MAY 12 TO SUNDAY, MAY 13 Both radio broadcasts and Washington State Department of Transportation (WSDOT to thems in the know) have been warning of closed interstate lanes, construction delays, and other impediments to our getting to the airport in reasonable tim e. Our flight to London is scheduled to depart Seattle at 1:20 this afternoon. My worrywart spouse wants to leave our apartment at 9:30 for what would ordinarily be a 45- minute drive. After some intense negotiation, we close our apartment door and head to the garage at 9:45. One thing that WSDOT did not (and could not) anticipate is that our 2017 Honda Accord would behave strangely as we approached it. The door locks opened/closed/opened/closed ... as we approached the inanimate beast. Surely it wasn't expressing its dismay at our leaving it for nearly three weeks? The two remote keys seemed to be behaving normally, but Lee was worried that we might not be able to get our luggage out of the trunk when we reached the airport. So she put hers on the back seat, but I (bravely) used the trunk for mine. The other concern is that we might not be able to lock the car at the airport, but this turned out not to be an issue. Well, we reached SEATAC's parking lot in 45 minutes (as I had predicted!) and entered the spiral ramps leading the various levels. On the electronic board, Level 5 was showing 300 spaces available to the left of the ramp. -

Breakfast Menu 7Am to 9.30Am, Monday to Friday
Breakfast Menu 7am to 9.30am, Monday to Friday. 8am to 10am, Saturday and Sunday. Served in the restaurant, lounge and outdoor seating area. Please see a member of our breakfast team to be seated Lighter Breakfasts e very best for your breakfast Eggs Benedict ... £5.50 In keeping with the food philosophy at Stirk House, with our own roast ham and buttered breakfast mun our breakfast ingredients are sourced from the very best Bacon sandwich ... £3.50 local artisan producers and provide a true taste of Lancashire Sausage sandwich ... £3.50 Baked beans on toast ... £3.50 Cooked Breakfasts Pair of boiled eggs with soldiers ... £3.50 Hot buttered crumpets ... £3.50 All breakfast items are cooked to order. Please order your breakfast as you require, with any variation on the suggestions below. Healthy Breakfast Options Stirk House ‘Lancashire Breakfast’ ... £7.95 Homemade porridge ... £3.50 John Penny’s pork sausage and black pudding, best back bacon, grilled tomato, grilled mushroom, Homemade granola ... £3.50 fried free-range egg and pan-fried bread with red fruits and Greek yoghurt Stirk House vegetarian breakfast ... £5.95 Bloody Mary Grilled tomato, Glamorgan sausage, baked beans, grilled mushrooms, fried free-range egg and pan-fried bread Vodka shot, lemon juice ... £6.50 Worcestershire sauce, Tabasco sauce ‘Port of Lancaster Smokehouse’ smoked salmon... £7.50 tomato juice, salt, freshly ground black pepper, with scrambled eggs stick of celery, with or without ice. Make it a double ... £7.50 ree egg Lancashire cheese ... £4.50 and spring onion omelette Make it a virgin (without the vodka) ..