An Assessment of Invasive Plant Species Monitored by the Northern Research Station Forest Inventory and Analysis Program, 2005 Through 2010
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Feedstock List (As of 3/2018)
Feedstock List (as of 3/2018) FOG: Fats / Oils / Greases Wastes / Oil Seeds Algae / Aquatic Species Industrial Aloe (Aloe vera) Meadowfoam (Limnanthes alba) Brown grease Cyanobacteria Babassu (Attalea speciosa) Mustard (Sinapis alba) Crude glycerine Halophytes (e.g., Salicornia bigelovii) *Camelina (Camelina sativa)* Nuts Fish oil Lemna (Lemna spp.) *Canola, winter (Brassica napus[occasionally rapa Olive (Olea europaea) Industrial effluent (palm) Macroalgae or campestris])* *Carinata (Brassica carinata)* Palm (Elaeis guineensis) Shrimp oil (Caridea) Mallow (Malva spp.) Castor (Ricinus communis) Peanut, Cull (Arachis hypogaea) Tall oil pitch Microalgae Citrus (Citron spp.) Pennycress (Thlaspi arvense) Tallow / Lard Spirodela (Spirodela polyrhiza) Coconut (Cocos nucifera) Pongamia (Millettia pinnata) White grease Wolffia (Wolffia arrhiza) Corn, inedible (Zea mays) Poppy (Papaver rhoeas) Waste vegetable oil Cottonseed (Gossypium) *Rapeseed (Brassica napus)* Yellow grease Croton megalocarpus Oryza sativa Croton ( ) Rice Bran ( ) Cuphea (Cuphea viscossisima) Safflower (Carthamus tinctorius) Flax / Linseed (Linum usitatissimum) Sesame (Sesamum indicum) Gourds / Melons (Cucumis melo) Soybean (Glycine max) Grapeseed (Vitis vinifera) Sunflower (Helianthus annuus) Hemp seeds (Cannabis sativa) Tallow tree (Triadica sebifera) Jojoba (Simmondsia chinensis) Tobacco (Nicotiana tabacum) Jatropha (Jatropha curcas) Calophyllum inophyllum Kamani ( ) Lesquerella (Lesquerella fenderi) Cellulose Woody Grasses Residues Other Types: Arundo (Arundo donax) Bagasse -

Bioenergy and Invasive Plants: Quantifying and Mitigating Future Risks
Invasive Plant Science and Management 2014 7:199–209 Bioenergy and Invasive Plants: Quantifying and Mitigating Future Risks Jacob N. Barney* The United States is charging toward the largest expansion of agriculture in 10,000 years with vast acreages of primarily exotic perennial grasses planted for bioenergy that possess many traits that may confer invasiveness. Cautious integration of these crops into the bioeconomy must be accompanied by development of best management practices and regulation to mitigate the risk of invasion posed by this emerging industry. Here I review the current status of United States policy drivers for bioenergy, the status of federal and state regulation related to invasion mitigation, and survey the scant quantitative literature attempting to quantify the invasive potential of bioenergy crops. A wealth of weed risk assessments are available on exotic bioenergy crops, and generally show a high risk of invasion, but should only be a first-step in quantifying the risk of invasion. The most information exists for sterile giant miscanthus, with preliminary empirical studies and demographic models suggesting a relatively low risk of invasion. However, most important bioenergy crops are poorly studied in the context of invasion risk, which is not simply confined to the production field; but also occurs in crop selection, harvest and transport, and feedstock storage. Thus, I propose a nested-feedback risk assessment (NFRA) that considers the entire bioenergy supply chain and includes the broad components of weed risk assessment, species distribution models, and quantitative empirical studies. New information from the NFRA is continuously fed back into other components to further refine the risk assessment; for example, empirical dispersal kernels are utilized in landscape-level species distribution models, which inform habitat invasibility studies. -

Chinese Tallow Tree (Triadica Sebifera)
THE WEEDY TRUTH ABOUT BIOFUELS TIM LOW & CAROL BOOTH Invasive Species Council October 2007 Title: The Weedy Truth About Biofuels Authors: Tim Low & Carol Booth Published by the Invasive Species Council, Melbourne October 2007 Updated March 2008 The INVASIVE SPECIES COUNCIL is a non-government organisation that works to protect the Australian environment from invasive pest species. Address: PO Box 166, Fairfield, Vic 3078 Email: [email protected] Website: www.invasives.org.au Further copies of this report can be obtained from the ISC website at www.invasives.org.au Cover photo: Spartina alterniflora, by the US Department of Agriculture CCOONNTTEENNTTSS Introduction ............................................................................................................................ 1 What are biofuels? ................................................................................................................ 2 The Biofuel industry .............................................................................................................. 4 The problems with biofuels ................................................................................................ 6 Social and economic issues ............................................................................................ 6 Greenhouse issues ............................................................................................................ 7 Biodiversity issues ........................................................................................................... -

Chinese Tallow Invasion in Maritime Forests: Understand Invasion Mechanism and Develop Ecologically-Based Management Lauren Susan Pile Clemson University
Clemson University TigerPrints All Dissertations Dissertations 12-2015 Chinese Tallow Invasion in Maritime Forests: Understand Invasion Mechanism and Develop Ecologically-Based Management Lauren Susan Pile Clemson University Follow this and additional works at: https://tigerprints.clemson.edu/all_dissertations Part of the Forest Sciences Commons Recommended Citation Pile, Lauren Susan, "Chinese Tallow Invasion in Maritime Forests: Understand Invasion Mechanism and Develop Ecologically-Based Management" (2015). All Dissertations. 1807. https://tigerprints.clemson.edu/all_dissertations/1807 This Dissertation is brought to you for free and open access by the Dissertations at TigerPrints. It has been accepted for inclusion in All Dissertations by an authorized administrator of TigerPrints. For more information, please contact [email protected]. CHINESE TALLOW INVASION IN MARITIME FORESTS: UNDERSTAND INVASION MECHANISM AND DEVELOP ECOLOGICALLY-BASED MANAGEMENT A Dissertation Presented to the Graduate School of Clemson University In Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy Forest Resources by Lauren Susan Pile December 2015 Accepted by: G. Geoff Wang, Committee Chair William C. Bridges Jr. Patricia A. Layton Thomas A. Waldrop Joan L. Walker ABSTRACT Invasion by highly aggressive, non-native, invasive plants is a significant threat to management and conservation priorities as these plants can transform ecosystem functions and processes. In this study, I investigated the non-native, invasive tree, Chinese tallow -

2020 Invasive Species Update
Quiet Invasion: 2020 Invasive Species Update Lisa Gonzalez Invasive Species: The Continuing Problem • New species being reported • Invasive species management is multi‐ faceted and long‐term • Few eradication success stories Policy Research Management Education & & Awareness Restoration What We Know Common Characteristics Impacts • fast seed germination • nutrient cycling • high population growth • local hydrology • early reproductive maturity • fire regimes • reproduction vegetatively as well as sexually • geomorphological processes (such as dune formation or stream profile) • generalized pollination • species and structural diversity • wide tolerance to many habitat types • disease • adaptation to disturbance • impacts available wildlife resources • high rate of biomass accumulation • prevents recruitment of native species due to • long‐range seed dispersal capabilities competition for light, nutrients, and/or moisture • fruit used by wildlife (including humans) • economic losses and costs • relative lack of predators or diseases in • sense of place and quality of life present location Pathways of Introduction • Landscaping and horticulture • Mowing equipment and soils • Shipping materials • Aquarium trade and aquarists • Shipping and boating • Agriculture and livestock • Internet sales • Live seafood markets, bait • Biological control • Scientific research institutions, public aquaria, zoos, arboreta, wildlife preserves Longstanding Invaders • Giant reed Arundo donax • Yellow bluestem Bothriochloa ischaemum • Japanese honeysuckle Lonicera -

Chinese Tallowtree and Carolina Ash Seedlings
Species: Triadica sebifera Page 1 of 30 SPECIES: Triadica sebifera z Introductory z Distribution and occurrence z Botanical and ecological characteristics z Fire ecology z Fire effects z Management considerations z References INTRODUCTORY SPECIES: Triadica sebifera z AUTHORSHIP AND CITATION z FEIS ABBREVIATION z SYNONYMS z NRCS PLANT CODE z COMMON NAMES z TAXONOMY z LIFE FORM z FEDERAL LEGAL STATUS z OTHER STATUS Jeff Hutchison, Archbold Biological Station AUTHORSHIP AND CITATION: Meyer, Rachelle. 2005. Triadica sebifera. In: Fire Effects Information System, [Online]. U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory (Producer). Available: http://www.fs.fed.us/database/feis/ [ ]. FEIS ABBREVIATION: TRISEB SYNONYMS: Sapium sebiferum (L.) Roxb. [36,88,132,134] NRCS PLANT CODE [129]: TRSE6 COMMON NAMES: tallowtree Chinese tallow popcorn tree Florida aspen chicken tree http://www.fs.fed.us/database/feis/plants/tree/triseb/all.html 9/26/2007 Species: Triadica sebifera Page 2 of 30 TAXONOMY: The scientific name of tallowtree is Triadica sebifera (L.) Small (Euphorbiaceae) [30,57,129]. LIFE FORM: Tree FEDERAL LEGAL STATUS: None OTHER STATUS: Tallowtree is considered a noxious weed in Florida. Its sale there was prohibited in 1998 [127]. The Southern Region of the Forest Service has listed it as a Category 1 weed species [128]. It is also included in the top 10 exotic pest plants in Georgia by the Georgia Exotic Pest Plant Council [34] and listed as a "red alert" species in California by the California Invasive Pest Plant Council [9]. DISTRIBUTION AND OCCURRENCE SPECIES: Triadica sebifera z GENERAL DISTRIBUTION z ECOSYSTEMS z STATES/PROVINCES z BLM PHYSIOGRAPHIC REGIONS z KUCHLER PLANT ASSOCIATIONS z SAF COVER TYPES z SRM (RANGELAND) COVER TYPES z HABITAT TYPES AND PLANT COMMUNITIES Cheryl McCormick, The University of Georgia, IPM Images GENERAL DISTRIBUTION: Tallowtree is a native of China and Japan [29,68,69,76,131,134]. -

Plant-Based (Camelina Sativa) Biodiesel Manufacturing Using The
Plant-based (Camelina Sativa) biodiesel manufacturing using the technology of Instant Controlled pressure Drop (DIC) : process performance and biofuel quality Fanar Bamerni To cite this version: Fanar Bamerni. Plant-based (Camelina Sativa) biodiesel manufacturing using the technology of In- stant Controlled pressure Drop (DIC) : process performance and biofuel quality. Chemical and Process Engineering. Université de La Rochelle, 2018. English. NNT : 2018LAROS004. tel-02009827 HAL Id: tel-02009827 https://tel.archives-ouvertes.fr/tel-02009827 Submitted on 6 Feb 2019 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NIVERSITÉ DE LA ROCHELLE UFR des SCIENCES et TECHNOLOGIE Année: 2018 Numéro attribué par la bibliothèque: THÈSE pour obtenir le grade de DOCTEUR de L’UNIVERSITÉ DE LA ROCHELLE Discipline : Génie des Procédés Industriels Présentée et soutenue par Fanar Mohammed Saleem Amin BAMERNI Le 23 février 2018 TITRE: Procédé de Fabrication de Biodiesel assistée par Texturation par Détente Instantanée Contrôlée (DIC) de Camelina Sativa : Performance des Procédés et Qualité du Produit. Plant-Based (Camelina Sativa) Biodiesel Manufacturing Using the Technology of Instant Controlled Pressure Drop (DIC); Process performance and biofuel Quality. Dirigée par : Professeur Ibtisam KAMAL et Professeur Karim ALLAF JURY: Rapporteurs: M. -

Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona Muricata L.) Seed Oil
energies Article Biodiesel Production from a Novel Nonedible Feedstock, Soursop (Annona muricata L.) Seed Oil Chia-Hung Su *, Hoang Chinh Nguyen, Uyen Khanh Pham, My Linh Nguyen and Horng-Yi Juan Graduate School of Biochemical Engineering, Ming Chi University of Technology, New Taipei City 24301, Taiwan; [email protected] (H.C.N.); [email protected] (U.K.P.); [email protected] (M.L.N.), [email protected] (H.-Y.J.) * Correspondence: [email protected]; Tel.: +886-22-908-9899 (ext. 4665) Received: 11 August 2018; Accepted: 24 September 2018; Published: 26 September 2018 Abstract: This study investigated the optimal reaction conditions for biodiesel production from soursop (Annona muricata) seeds. A high oil yield of 29.6% (w/w) could be obtained from soursop seeds. Oil extracted from soursop seeds was then converted into biodiesel through two-step transesterification process. A highest biodiesel yield of 97.02% was achieved under optimal ◦ acid-catalyzed esterification conditions (temperature: 65 C, 1% H2SO4, reaction time: 90 min, and a methanol:oil molar ratio: 10:1) and optimal alkali-catalyzed transesterification conditions (temperature: 65 ◦C, reaction time: 30 min, 0.6% NaOH, and a methanol:oil molar ratio: 8:1). The properties of soursop biodiesel were determined and most were found to meet the European standard EN 14214 and American Society for Testing and Materials standard D6751. This study suggests that soursop seed oil is a promising biodiesel feedstock and that soursop biodiesel is a viable alternative to petrodiesel. Keywords: Annona muricata; biodiesel production; seed oil; soursop; two-step process 1. -

Quarantine Host Range and Natural History of Gadirtha Fusca, a Potential Biological Control Agent of Chinese Tallowtree (Triadica Sebifera) in North America
DOI: 10.1111/eea.12737 Quarantine host range and natural history of Gadirtha fusca, a potential biological control agent of Chinese tallowtree (Triadica sebifera) in North America Gregory S. Wheeler1* , Emily Jones1, Kirsten Dyer1, Nick Silverson1 & Susan A. Wright2 1USDA/ARS Invasive Plant Research Laboratory, 3225 College Ave., Ft Lauderdale, FL 33314, USA, and 2USDA/ARS Invasive Plant Research Laboratory, Gainesville, FL 32608, USA Accepted: 23 August 2018 Key words: biocontrol, classical biological control, weed control, Euphorbiaceae, defoliating caterpillar, host range tests, invasive weeds, Sapium, Lepidoptera, Nolidae, integrated pest management, IPM Abstract Classical biological control can provide an ecologically sound, cost-effective, and sustainable manage- ment solution to protect diverse habitats. These natural and managed ecosystems are being invaded and transformed by invasive species. Chinese tallowtree, Triadica sebifera (L.) Small (Euphorbiaceae), is one of the most damaging invasive weeds in the southeastern USA, impacting wetlands, forests, and natural areas. A defoliating moth, Gadirtha fusca Pogue (Lepidoptera: Nolidae), was discovered feeding on Chinese tallowtree leaves in the weed’s native range and has been tested for its suitability as a biological control agent. Natural history studies of G. fusca indicated that the neonates have five instars and require 15.4 days to reach pupation. Complete development from egg hatch to adult emergence required 25.8 days. No differences were found between males and females in terms of life history and nutritional indices measured. Testing of the host range of G. fusca larvae was conducted with no-choice, dual-choice, and multigeneration tests and the results indicated that this species has a very narrow host range. -
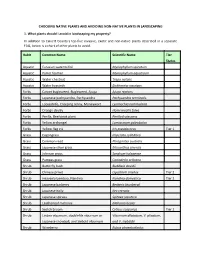
Choosing Native Plants and Avoiding Non-Native Plants in Landscaping
CHOOSING NATIVE PLANTS AND AVOIDING NON-NATIVE PLANTS IN LANDSCAPING 1. What plants should I avoid in landscaping my property? In addition to Calvert County’s top-five invasive, exotic and non-native plants described in a separate FAQ, below is a chart of other plants to avoid. Habit Common Name Scientific Name Tier Status Aquatic Eurasian watermilfoil Myriophyllum spicatum Aquatic Parrot feather Myriophyllum aquaticum Aquatic Water chestnut Trapa natans Aquatic Water hyacinth Eichhornia crassipes Forbs Carpet bugleweed, Bugleweed, Ajuga Ajuga reptans Forbs Japanese pachysandra, Pachysandra Pachysandra terminalis Forbs Loosestrife, Creeping Jenny, Moneywort Lysimachia nummularia Forbs Orange daylily Hemerocallis fulva Forbs Perilla, Beefsteak plant Perilla frutescens Forbs Yellow archangel Lamiastrum galeobolon Forbs Yellow flag iris Iris pseudacorus Tier 1 Grass Cogongrass Imperata cylindrica Grass Common reed Phragmites australis Grass Japanese silver grass Miscanthus sinensis Grass Johnson grass Sorghum halepense Grass Pampas grass Cortaderia selloana Shrub Butterfly bush Buddleia davidii Shrub Chinese privet Ligustrum sinense Tier 2 Shrub Heavenly bamboo, Nandina Nandina domestica Tier 2 Shrub Japanese barberry Berberis thunbergii Shrub Japanese holly Ilex crenata Shrub Japanese spiraea Spiraea japonica Shrub Leatherleaf mahonia Mahonia bealei Shrub Scotch broom Cytisus scoparius Tier 2 Shrub Linden viburnum, doublefile viburnum or Viburnum dilatatum, V. plicatum, Japanese snowball, and Siebold viburnum and V. sieboldii Shrub Wineberry Rubus phoenicolasius Shrub Winged euonymus Euonymus alatus Tier 2 Tree Amur corktree, Phellodendron Phellodendron amurense Tree Bradford pear Pyrus calleryana Tier 2 Tree Chinese tallow Triadica sebifera Tree Goldenrain tree Koelreuteria paniculata Tree Norway maple Acer platanoides Tree Sawtooth oak Quercus acutissima Vine Chinese wisteria, Japanese wisteria Wisteria sinensis, W. -

Growing Risk: Addressing the Invasive Potential of Bioenergy Feedstocks 1 Growing Risk Addressing the Invasive Potential of Bioenergy Feedstocks
Growing Risk Addressing the Invasive Potential of Bioenergy Feedstocks Aviva Glaser and Patty Glick 2012 Growing Risk: Addressing the Invasive Potential of Bioenergy Feedstocks 1 Growing Risk Addressing the Invasive Potential of Bioenergy Feedstocks Prepared by Aviva Glaser, Legislative Representative, Agriculture Policy Patty Glick, Senior Climate Change Specialist Acknowledgements This report was made possible due to the generous support of the Doris Duke Charitable Foundation. The authors wish to thank many people for their time and contributions to this report. We would like to thank the following National Wildlife Federation staff for providing valuable edits and feedback: Julie Sibbing, Bruce Stein, Doug Inkley, and Lara Bryant. Additionally, we would like to thank several experts for their time, input, and helpful review comments: Dr. Joseph DiTomaso, University of California, Davis; Dr. Doria Gordon, The Nature Conservancy; Bryan Endres, J.D., University of Illinois; Dr. Lauren Quinn, University of Illinois; Doug Johnson, California Invasive Plant Council; and Read Porter, J.D., Environmental Law Institute. Designed by Maja Smith, MajaDesign, Inc. © 2012 National Wildlife Federation Cover image: The highly-invasive giant reed (Arundo donax), a candidate species for bioenergy production, has taken over vast areas along the Rio Grande, as seen in this aerial view near Eagle Pass, Texas. Credit: John Goolsby, USDA. Suggested citation: Glaser, A. and P. Glick. 2012. Growing Risk: Addressing the Invasive Potential of Bioenergy Feedstocks. Washington, DC: National Wildlife Federation. i Growing Risk: Addressing the Invasive Potential of Bioenergy Feedstocks Table of Contents Little bluestem, a native grass. Credit: NRCS. 1. Executive Summary 1 2. Overview 3 The Promise of Bioenergy 3 Risks from Bioenergy 4 A Focus on Invasiveness 4 3. -

Programmatic Environmental Assessment for Rural Development’S Proposed Rules Related to Biofuels Development
Programmatic Environmental Assessment For Rural Development’s Proposed Rules Related to Biofuels Development Prepared by: USDA Rural Development Rural Business Programs 1400 Independence Ave. S.W. Washington, DC 20250 August 4, 2010 Table of Contents 1. Purpose and Need for Proposed Rules 3 2. Proposed Action 3 2.1 Background 3 2.2 Proposed Rules 5 3.1 Alternatives 19 3.2 No Action 19 4.0 Environmental Impacts of Proposed Action and Alternatives 20 4.1 Impacts of Proposed Rule for Section 9003 Program 20 4.2 Impacts of Proposed Rule for Section 9004 Program 23 4.3 Impacts of Proposed Rule for Section 9005 Program 24 4.4 Cumulative Impacts 31 4.5 Impacts of Alternative 1 41 4.6 Impacts of No Action Alternative 42 5. List of Agencies and Persons Consulted 44 6. References 44 Appendix A: 2008 Farm Bill Renewable Energy Provisions 46 Appendix B: Current Advanced Biofuel Projects with DOE Funding 48 Appendix C: Advanced Biofuels Investments (2009-2010) 52 Appendix D: Types of Feedstocks tested for Biodiesel 54 Appendix E: Issues in Cellulosic Biomass Commercialization 56 Appendix F: Operating Biodiesel Plants (2009) 58 Appendix G: Advanced Biofuels Payments (FY 2009) 62 2 1. Purpose and Need for Proposed Action Rural Development (the Agency) proposes to adopt final rules to administer the biofuels programs enacted by Title IX of the Food, Conservation, and Energy Act of 2008. The purposes of the rules are to provide for financial support related to the development of renewable energy and advanced biofuels in the U.S. 2. Proposed Action 2.1 Background Rural Development (the Agency) administers a multitude of Federal programs for the benefit of rural America, ranging from housing and community facilities to infrastructure and business development.