Download This Document
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Report on the Joint World Heritage Centre / ICOMOS Advisory Mission to Stonehenge, Avebury and Associated Sites
World Heritage 41 COM Patrimoine mondial Paris, 27 June / 27 juin 2017 Original: English UNITED NATIONS EDUCATIONAL, SCIENTIFIC AND CULTURAL ORGANIZATION ORGANISATION DES NATIONS UNIES POUR L'EDUCATION, LA SCIENCE ET LA CULTURE CONVENTION CONCERNING THE PROTECTION OF THE WORLD CULTURAL AND NATURAL HERITAGE CONVENTION CONCERNANT LA PROTECTION DU PATRIMOINE MONDIAL, CULTUREL ET NATUREL WORLD HERITAGE COMMITTEE / COMITE DU PATRIMOINE MONDIAL Forty-first session / Quarante-et-unième session Krakow, Poland / Cracovie, Pologne 2-12 July 2017 / 2-12 juillet 2017 Item 7 of the Provisional Agenda: State of conservation of properties inscribed on the World Heritage List and/or on the List of World Heritage in Danger Point 7 de l’Ordre du jour provisoire: Etat de conservation de biens inscrits sur la Liste du patrimoine mondial et/ou sur la Liste du patrimoine mondial en péril MISSION REPORT / RAPPORT DE MISSION Stonehenge, Avebury and Associated Sites (United Kingdom of Great Britain and Northern Ireland) (373bis) Stonehenge, Avebury et sites associés (Royaume-Uni de Grande-Bretagne et d'Irlande du Nord) (373bis) 31 January – 3 February 2017 Report on the joint World Heritage Centre / ICOMOS Advisory Mission to Stonehenge, Avebury and Associated sites 31 January – 3 February 2017 Table of contents Executive Summary 1. Introductory Statements 1.1 Acknowledgments 1.2. Aims and mandate of the February 2017 Mission 2. Context and background 2.1 Statement of Outstanding Universal Value (OUV) 2.2 Summary 1st Mission recommendations (October 2015 – report April 2016). 2.3 Reactions by the civil society 2.4 Governance and consensus building among heritage bodies 3. Responses by the SP to the recommendations of the first Mission - April 2016 3.1 Willingness to respond 3.2 Issues of archaeological organisation and quality control 3.3 Issue of visitor numbers and behaviour 4. -

February 2019
Contents / Diary of events FEBRUARY 2019 Bristol Naturalist News Picture © Alex Morss Discover Your Natural World Bristol Naturalists’ Society BULLETIN NO. 577 FEBRUARY 2019 BULLETIN NO. 577 FEBRUARY 2019 Bristol Naturalists’ Society Discover Your Natural World Registered Charity No: 235494 www.bristolnats.org.uk CONTENTS HON. PRESIDENT: Andrew Radford, Professor 3 Diary of Events of Behavioural Ecology, Bristol University Nature in Avon – submissions invited; HON. CHAIRMAN: Ray Barnett [email protected] 4 Society Winter Lecture; HON. PROCEEDINGS RECEIVING EDITOR: Subs. due; Welcome new members Dee Holladay, [email protected] ON EC 5 Society AGM ; Bristol Weather H . S .: Lesley Cox 07786 437 528 [email protected] 6 Natty News: Fossil evidence on evolution HON. MEMBERSHIP SEC: Mrs. Margaret Fay of feathers & colour vision; volcanic doubts; Beech 81 Cumberland Rd., BS1 6UG. 0117 921 4280 disease; migrant murder; Fin whales [email protected] 8 BOTANY SECTION HON. TREASURER: Mary Jane Steer 01454 294371 [email protected] Botanical notes ; Talk Report; Field Mtg Reports; Libby Houston’s BULLETIN COPY DEADLINE: 7th of month before new honour (p10); Plant Records publication to the editor: David B Davies, 51a Dial Hill Rd., Clevedon, BS21 7EW. 12 GEOLOGY SECTION 01275 873167 [email protected] 13 INVERTEBRATE SECTION . Notes for February; Health & Safety on walks: Members Wildlife Photographer 2018 participate at their own risk. They are 14 LIBRARY Books to give away responsible for being properly clothed and shod. Dogs may only be brought on a walk with prior 16 ORNITHOLOGY SECTION agreement of the leader. Meeting Reports; 18 Breeding Bird Survey; Recent News 19 MISCELLANY Botanic Garden Avon Organic Group 20 Gorge & Downs Wildlife Project; Cover picture:: Twenty of the 48 plants found in flower on New Year’s Day. -

NORTH SOMERSET COUNCIL Green Infrastructure Strategy
SUMMARY JANUARY 2021 NORTH SOMERSET COUNCIL Green Infrastructure Strategy Parks Beaches Green spaces Countryside Waterways Wildlife 2 Our parks, beaches, green spaces, wildlife, countryside, public rights of way and waterways are precious, and we have prepared this new strategy to help to protect and enhance them. 3 Green Infrastructure Strategy Contents What is Green Infrastructure? 5 Why do we need a strategy? 6 Aims, vision and objectives 8 Our green infrastructure 10 Green infrastructure opportunities 15 Action Plan 27 The information in this summary document has been drawn from a much wider piece of work that includes the data which underpins the maps shown in this summary; a more detailed analysis of North Somerset’s GI; and wide ranging information about GI in general. It is the detailed report that will underpin the way the Council manages GI across North Somerset. View document > Summary January 2021 4 What is Green Infrastructure? Green infrastructure is a technical term that we use as shorthand to describe how we will look after these spaces. We have adopted this definition below to help explain in more detail what we mean. Green infrastructure is a strategically planned network of natural and semi-natural areas with other environmental features designed and managed to deliver a wide range of benefits (typically called ecosystem services) such as water purification, air quality, biodiversity, space for recreation and climate This network of green (land) and blue (water) spaces can improve mitigation and adaptation. environmental conditions and therefore citizens’ health and quality of life. It also supports a green economy, creates job opportunities and enhances biodiversity. -

A303 Stonehenge Amesbury to Berwick Down Technical Appraisal Report Volume 3 Appendix B Initial Corridors Appraisal (Design Fix A)
A303 Stonehenge Amesbury to Berwick Down Technical Appraisal Report Volume 3 Appendix B Initial corridors appraisal (Design Fix A) Public Consultation 2017 A303 Amesbury to Berwick Down | HE551506 Appendix B Initial corridors appraisal (Design Fix A) A303 Amesbury to Berwick Down | HE551506 B.1 Historical routes A360 LONGBARROW ROUNDABOUT A345 COUNTESS A3028 ROUNDABOUT DURRINGTON LARKHILL SHREWTON A303 B3086 A3028 STONEHENGE WINTERBOURNE STOKE RIVER TILL RIVER A303 AMESBURY YARNBURY CASTLE BERWICK DOWN A303 BERWICK ST JAMES BOSCOMBE DOWN AIRFIELD A345 A360 RIVER AVON A36 STAPLEFORD UPPER WOODFORD MIDDLE WOODFORD LOWER WOODFORD SALISBURY Kilometres 0 4.5 9 This map is reproduced from Ordnance Survey material with the permission of Ordnance Survey on behalf of the controller of Her Majesty's Stationery Office. © Crown Copyright. Unauthorised reproduction infringes Crown copyright and may lead to prosecution or civil proceedings. CLIENT NAME: Highways England LICENCE NUMBER: 100030649 [2016] © Historic England [2016]. Contains Ordnance Survey data © Crown copyright and database right [2016] The Historic England GIS Data contained in this material was obtained in 2016. The most publicly available up to date Historic England GIS Data can be obtained from HistoricEngland.org.uk. Drawing Status Suitability Project Title LEGEND 2004 Berkley-Matthews SAFETY, HEALTH AND ENVIRONMENTAL 1991_1993 S1 1991_1993 N1 1999-Winterbourne FIT FOR INTERNAL REVIEW AMND COMMENT S3 A303 AMESBURY TO BERWICK DOWN Route 2006 Northern Route INFORMATION 1991_1993 S1(B) -
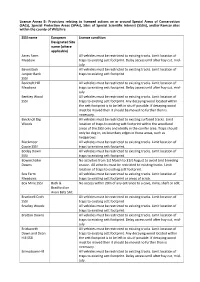
Annex B – Conditions Relating to Licensed Badger Control On
Licence Annex B: Provisions relating to licensed actions on or around Special Areas of Conservation (SACs), Special Protection Areas (SPAs), Sites of Special Scientific Interest (SSSIs), and/or Ramsar sites within the county of Wiltshire SSSI name European Licence condition Designated Site name (where applicable) Acres Farm All vehicles must be restricted to existing tracks. Limit location of Meadow traps to existing sett footprint. Delay access until after hay cut, mid- July. Baverstock All vehicles must be restricted to existing tracks. Limit location of Juniper Bank traps to existing sett footprint. SSSI Bencroft Hill All vehicles must be restricted to existing tracks. Limit location of Meadows traps to existing sett footprint. Delay access until after hay cut, mid- July. Bentley Wood All vehicles must be restricted to existing tracks. Limit location of SSSI traps to existing sett footprint. Any decaying wood located within the sett footprint is to be left in situ if possible. If decaying wood must be moved then it should be moved no further than is necessary. Bincknoll Dip All vehicles must be restricted to existing surfaced tracks. Limit Woods location of traps to existing sett footprint within the woodland areas of the SSSI only and ideally in the conifer area. Traps should only be dug in, on boundary edges in these areas, such as hedgerows. Blackmoor All vehicles must be restricted to existing tracks. Limit location of Copse SSSI traps to existing sett footprint. Botley Down All vehicles must be restricted to existing tracks. Limit location of SSSI traps to existing sett footprint. Bowerchalke No activities from 1st March to 31st August to avoid bird breeding Downs season. -

Ecosystems Woodlands Fieldwork
Woodland ecosystems and their management Embedding fieldwork into the curriculum Woodlands fieldwork can add value to a range of topics including: • Tourism and leisure • National Parks • Woodland management • Environmental conservation • Ecosystems • Conflicts of interest • Economic use of woodlands • Comparing types and ages of woodlands • Vegetation species • Microclimates There are several cross curricular themes such as: • Geography units such as unit 16 'What is development?', in terms of resource use • ICT, including using a mapping package, using internet search engines • Citizenship thorough conflicts of interest, considering topical issues, justifying personal opinion • Science in terms of habitats, toxic materials in food chains and environmental chemistry • Geography units such as unit 8 'Coastal environments' and unit 13 'Limestone landscapes of England' in terms of human impacts on natural areas and pressures of tourism • key skills, working with others, improving own learning and performance • PSHE in terms of taking responsibility for own actions • Enquiries and decision making preparation for GCSE and A level QCA unit schemes available to download for: Geography http://www.standards.dfes.gov.uk/schemes2/secondary_geography/?view=get Science: http://www.standards.dfes.gov.uk/schemes2/secondary_science/?view=get Citizenship http://www.standards.dfes.gov.uk/schemes2/citizenship/?view=get Accompanying scheme of work The scheme of work below has been created using aspects from a variety of QCA units and schemes available, including: Unit 14: Can the earth cope? Ecosystems, population and resources http://www.standards.dfes.gov.uk/schemes2/secondary_geography/geo14/?view=get Unit 23: Local action, global effects http://www.standards.dfes.gov.uk/schemes2/secondary_geography/geo23/?view=get Royal Geographical Society with the Institute of British Geographers © Woodland Ecosystems About the unit The unit is adapted from the QCA scheme of work Unit 14 Can the earth cope? Ecosystems population and resources. -

SSSI) Notified Under Section 28 of the Wildlife and Countryside Act, 1981, As Amended
COUNTY: HAMPSHIRE/DORSET/WILTSHIRE SITE NAME: RIVER AVON SYSTEM Status: Site of Special Scientific Interest (SSSI) notified under Section 28 of the Wildlife and Countryside Act, 1981, as amended. Environment Agency Region: South Wessex Water Company: Wessex Water PLC, Bournemouth and West Hampshire Water Company Local Planning Authorities: Hampshire County Council, Dorset County Council, Wiltshire County Council, East Dorset District Council, New Forest District Council, Christchurch Borough Council, Salisbury District Council, Kennet District Council, West Wilts District Council National Grid Reference: SZ 163923 (Christchurch Harbour) to: SU 073583 (Avon) ST 867413 (Wylye) ST 963297 (Nadder), SU 170344 (Bourne) SZ 241147 (Dockens Water) Extent of River SSSI: Approx 205.11 km, 507.79 (ha.) Ordnance Survey Sheet: (1:50 000) 173 183 184 195 Date notified (under 1981 Act): 16 December 1996 Other Information: A new river SSSI. This site is listed as Grade 1* quality in ‘A Nature Conservation Review’ edited by D A Ratcliffe, C.U.P., 1977. Parts of the site are separately notified as: Lower Woodford Water Meadows SSSI (1987); East Harnham Meadows (1995); Britford Water Meadows (1987); Avon Valley (Bickton- Christchurch) SSSI (1993). The site is significant for the following habitat and species covered by Council Directive 92/43/EEC on The Conservation of Natural Habitats and of Wild Flora and Fauna: Habitat Floating vegetation of Ranunculus of plain and submountainous rivers Species Sea Lamprey Petromyzon marinus Annex IIa Brook lamprey Lampetra planeri Annex IIa Atlantic salmon Salmo salar Annex IIa, Va Bullhead Cotto gobius Annex IIa Desmoulin’s Whorl Snail Vertigo moulinsiana Annex IIa Parts of the site lie in the Avon Valley Environmentally Sensitive Area (ESA) and/or the West Wiltshire Downs and Cranborne Chase Area of Outstanding Natural Beauty (AONB). -

Screening Statement for the Isle of Wight LTP3 and Cross-Refers to Chapter Three of the Report
Habitats Regulations Assessments for The Isle of Wight LTP3: Volume 2 Site Descriptions, Qualifying Features and Conservation Objectives Client: Isle of Wight Council Report No.: UE-0076_IoWC_HRA_Vol2_4_081110HDnp Status: Final Date: November 2010 Author: HJD Checked: NEJP Approved: NJD CONTENTS CHAPTER I: INTRODUCTION 1 CHAPTER II: ECOLOGICAL DESCRIPTIONS 3 CHAPTER III: QUALIFYING FEATURES 15 CHAPTER IV: CONSERVATION OBJECTIVES 27 CHAPTER V: KEY ENVIRONMENTAL CONDITIONS SUPPORTING SITE INTEGRITY 69 UE Associates Ltd 2010 © This page is intentionally blank. UE Associates Ltd 2010 © HRA of the Isle of Wight Local Transport Plan 3 November 2010 UE-0076_IoWC_HRA_Vol2_4_081110HDnp Chapter I: Introduction 1.1 Purpose of this Document This document has been prepared by UE Associates on behalf of the Isle of Wight Council. It supports the HRA process by presenting information about all Special Areas of Conservation (SAC), Special Protection Areas (SPA) and Ramsar sites within 20km of the Isle of Wight. The sites are collectively referred to as European sites, and the following information is presented for each: An ecological description of the designated area; The qualifying features of the site (the reasons for which the site was designated); The site’s conservation objectives, where available; and Key environmental conditions that support the site’s integrity. The document accompanies the HRA Screening Statement for the Isle of Wight LTP3 and cross-refers to Chapter Three of the report. UE Associates Ltd 2010 © 1 HRA of the Isle of Wight -

Our Top 20 Reserves Access: Paths Can Be Muddy, Slippery and Steep-Sided
18 Weston Big Wood Grid ref: ST 452 750. Nearest postcode: BS20 8JY Weston Big Wood is one of Avon’s largest ancient woodlands. In springtime, the ground is covered with wood anemones, violets and masses of bluebells. Plants such as herb paris and yellow archangel together with the rare purple gromwell, show that this is an ancient woodland. The wood is very good for birds, including woodpecker, nuthatch, and tawny owl. Bats also roost in the trees and there are badger setts. Directions: From B3124 Clevedon to Portishead road, turn into Valley Road. Park in the lay-by approx 250 metres on right, and walk up the hill. Steps lead into the wood from the road. Our top 20 reserves Access: Paths can be muddy, slippery and steep-sided. Please keep away from the quarry sides. 19 Weston Moor Grid ref: ST 441 741. Nearest postcode: BS20 8PZ This Gordano Valley reserve has open moorland, species-rich rhynes, wet pasture and hay meadows. It is full of many rare plants such as cotton grass, marsh pennywort and lesser butterfly orchid, along with nationally scarce invertebrates such as the hairy dragonfly and ruddy darter. During the spring and summer the fields attract lapwing, redshank and snipe. Other birds such as little owl, linnet, reed bunting and skylark also breed in the area. Sparrowhawk, buzzard and green woodpecker are regularly recorded over the reserve. Directions: Parking is restricted and the approach to the reserve is hampered by traffic on the B3124 being particularly fast-moving. When parking please do not block entrances to farms, fields or homes. -

Bristol Naturalist News
Contents / Diary of events JULY-AUGUST 2019 Bristol Naturalist News Photo © Nick Owens Discover Your Natural World Bristol Naturalists’ Society BULLETIN NO. 582 JULY-AUGUST 2019 BULLETIN NO. 582 JULY-AUGUST 2019 Bristol Naturalists’ Society Discover Your Natural World Registered Charity No: 235494 www.bristolnats.org.uk CONTENTS HON. PRESIDENT: Andrew Radford, Professor 3 DIARY of Events; Nature in Avon; of Behavioural Ecology, Bristol University Welcome new members ON HAIRMAN H . C : Ray Barnett [email protected] 4 SOCIETY ITEMS: Mid-week Walks; HON. PROCEEDINGS RECEIVING EDITOR: Find a Bumblebee in Scotland; Dee Holladay, [email protected] ON EC 5 Bristol Weather H . S .: Lesley Cox 07786 437 528 [email protected] 6 NATTY NEWS : Bees, Feathers, HON. MEMBERSHIP SEC: Mrs. Margaret Fay 81 Cumberland Rd., BS1 6UG. 0117 921 4280 7 Bedbugs; Poisoned Birds; Fracking; Flock mechanics; Re-wilding; [email protected] HON. TREASURER: Mary Jane Steer 8 Duke of Burgundy; Fungus find 01454 294371 [email protected] 9 Westonbirt BioBlitz / BNS Survey BULLETIN COPY DEADLINE: 7th of month before publication to the editor: David B Davies, 10 BOTANY SECTION 51a Dial Hill Rd., Clevedon, BS21 7EW. ‘Other’ meetings; 01275 873167 [email protected] 11 Botanical notes ; Meeting Reports; . 13 Plant Records Health & Safety on walks: Members 15 GEOLOGY SECTION participate at their own risk. They are Book review: Dinosaurs Rediscovered responsible for being properly clothed and shod. Dogs may only be brought on a walk with prior 16 INVERTEBRATE SECTION agreement of the leader. Notes for June; Points of Interest 18 LIBRARY Books to give away; Donations; Steep Holm News 20 ORNITHOLOGY SECTION Sea Bird Safari; Swift nest petition; Meeting Report; Recent News ; 23 MISCELLANY Botanic Garden; 24 Gorge & Downs Wildlife Project; St George’s Flower Bank Fun-day Cover picture: The endangered Great Yellow Bumblebee – we are encouraged to find it in its retreat in the north of Scotland. -

Fitness for Purpose: English Nature's Use of Deliberative And
Fitness For Purpose: English Nature’s Use of Deliberative and Inclusionary Processes in the Delivery of Nature Conservation Policy. Katharine Louise Studd Department of Geography University College London. Thesis submitted in accordance with the requirements for the degree of PhD. University of London, 2003. ProQuest Number: U643316 All rights reserved INFORMATION TO ALL USERS The quality of this reproduction is dependent upon the quality of the copy submitted. In the unlikely event that the author did not send a complete manuscript and there are missing pages, these will be noted. Also, if material had to be removed, a note will indicate the deletion. uest. ProQuest U643316 Published by ProQuest LLC(2016). Copyright of the Dissertation is held by the Author. All rights reserved. This work is protected against unauthorized copying under Title 17, United States Code. Microform Edition © ProQuest LLC. ProQuest LLC 789 East Eisenhower Parkway P.O. Box 1346 Ann Arbor, Ml 48106-1346 Abstract The thesis explores the use of deliberative and inclusionary processes (DIPs) in nature conservation policy, using England’s statutory nature conservation advisor, English Nature, as the focus. While there is increasing pressure for publicly-funded organisations such as EN to incorporate DIPs into their operations, there is litde understanding within these organisations of how to apply these approaches in a way that is compatible with their responsibilities. The concept of Fitness for Purpose is presented as a structuring framework to explore English Nature’s use of DIPs in the context of its statutory responsibilities and institutionalised approaches, as well as the changing socio-political and conservation agendas in which the organisation is situated. -

Licence Annex B
LICENCE ANNEX B: Summary of all restrictions relating to licensed actions on Sites of Special Scientific Interest, Special Areas of Conservation, Special Protection Areas and RAMSAR Sites within the county of Wiltshire Protected Sites that are within the assessment are not necessarily part of any active operations. Active operations can and will only occur on protected sites where landowner permission has been granted. SSSI Site Name European Licence Conditions Site Name (if applicable) Acres Farm Meadow Restrict vehicles to existing tracks. Limit location of traps to existing sett footprint. Delay until after hay cut, mid July Bencroft Hill Restrict vehicles to existing tracks. Limit location of Meadows traps to existing sett footprint. Delay until after hay cut,mid July Bincknoll Dip Woods Restrict vehicles to existing surfaced tracks. Limit location of traps to within the woodland areas of the SSSI only and ideally in the conifer area. Traps should only be dug in, on boundary edges in these areas, such as hedgerows. Bowerchalke Downs Restrict vehicles to existing tracks. Limit location of traps to existing sett footprint Box Farm Meadows Restrict vehicles to existing tracks. Bradley Woods Restrict vehicles to existing tracks. Limit location of traps to existing sett footprint Bratton Downs Exclude SSSI or restrict vehicles to existing tracks. Limit location of traps to within the sett footprint or on improved/ semi-improved/scrub grassland areas. Limit location of traps to within the sett footprint which is already disturbed ground rhododendron or conifer plantation. Delay until after hay cut, mid-July. Brimsdown Hill Restrict vehicles to existing tracks. Limit location of traps to existing sett footprint.