ICD-9-CM Official Guidelines for Coding and Reporting
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Subtalar Joint Version 1.0 Effective June 15, 2021
CLINICAL GUIDELINES CMM-407: Arthroscopy: Subtalar Joint Version 1.0 Effective June 15, 2021 Clinical guidelines for medical necessity review of Comprehensive Musculoskeletal Management Services. © 2021 eviCore healthcare. All rights reserved. Comprehensive Musculoskeletal Management Guidelines V1.0 CMM-407: Arthroscopy: Subtalar Joint Definitions 3 General Guidelines 3 Indications 4 Non-Indications 5 Procedure (CPT®) Codes 5 References 6 ______________________________________________________________________________________________________ ©2021 eviCore healthcare. All Rights Reserved. Page 2 of 6 400 Buckwalter Place Boulevard, Bluffton, SC 29910 (800) 918-8924 www.eviCore.com Comprehensive Musculoskeletal Management Guidelines V1.0 Definitions Red flags indicate comorbidities that require urgent/emergent diagnostic imaging and/or referral for definitive therapy. Clinically meaningful improvement is defined as at least 50% improvement noted on global assessment. General Guidelines Either of the following are considered red flag conditions for subtalar joint arthroscopy: Post-reduction evaluation and management of the subtalar dislocation Septic arthritis in the subtalar joint Although imaging may be normal, prior to subtalar joint arthroscopy, radiographic imaging should be performed and include both of the following: Plain X-rays with one or more views (anteroposterior, lateral, axial, and/or Broden’s) to confirm and differentiate any of the following: Degenerative joint changes Loose bodies Osteochondral lesions Impingement -

In Patients with End-Stage Ankle Arthritis, How Does Total Ankle Arthroplasty Compared to Arthrodesis Affect Ankle Pain and Function?
University of the Pacific Scholarly Commons Physician's Assistant Program Capstones School of Health Sciences 4-1-2020 In Patients with End-Stage Ankle Arthritis, How Does Total Ankle Arthroplasty Compared to Arthrodesis Affect Ankle Pain and Function? Zachary Whipple University of the Pacific, [email protected] Follow this and additional works at: https://scholarlycommons.pacific.edu/pa-capstones Part of the Medicine and Health Sciences Commons Recommended Citation Whipple, Zachary, "In Patients with End-Stage Ankle Arthritis, How Does Total Ankle Arthroplasty Compared to Arthrodesis Affect Ankle Pain and Function?" (2020). Physician's Assistant Program Capstones. 84. https://scholarlycommons.pacific.edu/pa-capstones/84 This Capstone is brought to you for free and open access by the School of Health Sciences at Scholarly Commons. It has been accepted for inclusion in Physician's Assistant Program Capstones by an authorized administrator of Scholarly Commons. For more information, please contact [email protected]. In Patients with End-Stage Ankle Arthritis, How Does Total Ankle Arthroplasty Compared to Arthrodesis Affect Ankle Pain and Function? By Zachary Whipple Capstone Project Submitted to the Faculty of the Department of Physician Assistant Education of the University of the Pacific in partial fulfilment of the requirements for the degree of MASTER OF PHYSICIAN ASSISTANT STUDIES April 2020 Introduction End-stage ankle arthritis is a debilitating degenerative disease commonly located at the tibiotalar joint. The prevalence of symptomatic arthritis is about nine times lower than the rates associated with those of the knee or hip.1 Though less common than knee and hip arthritis, the US estimates greater than 50,000 new cases are reported each year.2 The most common etiology of ankle arthritis is post-traumatic pathology. -

Differences Between Subtotal Corpectomy and Laminoplasty for Cervical Spondylotic Myelopathy
Spinal Cord (2010) 48, 214–220 & 2010 International Spinal Cord Society All rights reserved 1362-4393/10 $32.00 www.nature.com/sc ORIGINAL ARTICLE Differences between subtotal corpectomy and laminoplasty for cervical spondylotic myelopathy S Shibuya1, S Komatsubara1, S Oka2, Y Kanda1, N Arima1 and T Yamamoto1 1Department of Orthopaedic Surgery, School of Medicine, Kagawa University, Kagawa, Japan and 2Oka Orthopaedic and Rehabilitation Clinic, Kagawa, Japan Objective: This study aimed to obtain guidelines for choosing between subtotal corpectomy (SC) and laminoplasty (LP) by analysing the surgical outcomes, radiological changes and problems associated with each surgical modality. Study Design: A retrospective analysis of two interventional case series. Setting: Department of Orthopaedic Surgery, Kagawa University, Japan. Methods: Subjects comprised 34 patients who underwent SC and 49 patients who underwent LP. SC was performed by high-speed drilling to remove vertebral bodies. Autologous strut bone grafting was used. LP was performed as an expansive open-door LP. The level of decompression was from C3 to C7. Clinical evaluations included recovery rate (RR), frequency of C5 root palsy after surgery, re-operation and axial pain. Radiographic assessments included sagittal cervical alignment and bone union. Results: Comparisons between the two groups showed no significant differences in age at surgery, preoperative factors, RR and frequency of C5 palsy. Progression of kyphotic changes, operation time and volumes of blood loss and blood transfusion were significantly greater in the SC (two- or three- level) group. Six patients in the SC group required additional surgery because of pseudoarthrosis, and four patients underwent re-operation because of adjacent level disc degeneration. -

Portal Hypertensionand Its Radiological Investigation
Postgrad Med J: first published as 10.1136/pgmj.39.451.299 on 1 May 1963. Downloaded from POSTGRAD. MED. J. (I963), 39, 299 PORTAL HYPERTENSION AND ITS RADIOLOGICAL INVESTIGATION J. H. MIDDLEMISS, M.D., F.F.R., D.M.R.D. F. G. M. Ross, M.B., B.Ch., B.A.O., F.F.R., D.M.R.D. From the Department of Radiodiagnosis, United Bristol Hospitals PORTAL hypertension is a condition in which there branch of the portal vein but may drain into the right is an blood in the branch. abnormally high pressure Small veins which are present on the serosal surface portal system of veins which eventually leads to of the liver and in the surrounding peritoneal folds splenomegaly and in chronic cases, to haematem- draining the diaphragm and stomach are known as esis and melaena. accessory portal veins. They may unite with the portal The circulation is in that it vein or enter the liver independently. portal unique The hepatic artery arises normally from the coeliac exists between two sets of capillaries, i.e. the axis but it may arise as a separate trunk from the aorta. capillaries of the spleen, pancreas, gall-bladder It runs upwards and to the right and divides into a and most of the gastro-intestinal tract on the left and right branch before entering the liver at the one hand and the sinusoids of the liver on the porta hepatis. The venous return starts as small thin-walled branches other hand. The liver parallels the lungs in that in the centre of the lobules in the liver. -

Procedure Coding in ICD-9-CM and ICD- 10-PCS
Procedure Coding in ICD-9-CM and ICD- 10-PCS ICD-9-CM Volume 3 Procedures are classified in volume 3 of ICD-9-CM, and this section includes both an Alphabetic Index and a Tabular List. This volume follows the same format, organization and conventions as the classification of diseases in volumes 1 and 2. ICD-10-PCS ICD-10-PCS will replace volume 3 of ICD-9-CM. Unlike ICD-10-CM for diagnoses, which is similar in structure and format as the ICD-9-CM volumes 1 and 2, ICD-10-PCS is a completely different system. ICD-10-PCS has a multiaxial seven-character alphanumeric code structure providing unique codes for procedures. The table below gives a brief side-by-side comparison of ICD-9-CM and ICD-10-PCS. ICD-9-CM Volume3 ICD-10-PCS Follows ICD structure (designed for diagnosis Designed and developed to meet healthcare coding) needs for a procedure code system Codes available as a fixed or finite set in list form Codes constructed from flexible code components (values) using tables Codes are numeric Codes are alphanumeric Codes are 3-4 digits long All codes are seven characters long ICD-9-CM and ICD-10-PCS are used to code only hospital inpatient procedures. Hospital outpatient departments, other ambulatory facilities, and physician practices are required to use CPT and HCPCS to report procedures. ICD-9-CM Conventions in Volume 3 Code Also In volume 3, the phrase “code also” is a reminder to code additional procedures only when they have actually been performed. -

Knee Arthrodesis After Failed Total Knee Arthroplasty
650 COPYRIGHT Ó 2019 BY THE JOURNAL OF BONE AND JOINT SURGERY,INCORPORATED Current Concepts Review Knee Arthrodesis After Failed Total 04/12/2019 on 1mhtSo9F6TkBmpGAR5GLp6FT3v73JgoS8Zn360/N4fAEQXu6c15Knc+cXP2J5+wvbQY2nVcoOF2DIk3Zd0BSqmOXRD8WUDFCPOJ9CnEHMNOmtIbs3S0ykA== by http://journals.lww.com/jbjsjournal from Downloaded Knee Arthroplasty Downloaded Asim M. Makhdom, MD, MSc, FRCSC, Austin Fragomen, MD, and S. Robert Rozbruch, MD from http://journals.lww.com/jbjsjournal Investigation performed at the Hospital for Special Surgery, Weill Cornell Medicine, Cornell University, New York, NY ä Knee arthrodesis after failure of a total knee arthroplasty (TKA) because of periprosthetic joint infection (PJI) may provide superior functional outcome and ambulatory status compared with above-the-knee amputation. by 1mhtSo9F6TkBmpGAR5GLp6FT3v73JgoS8Zn360/N4fAEQXu6c15Knc+cXP2J5+wvbQY2nVcoOF2DIk3Zd0BSqmOXRD8WUDFCPOJ9CnEHMNOmtIbs3S0ykA== ä The use of an intramedullary nail (IMN) for knee arthrodesis following removal of TKA components because of a PJI may result in higher fusion rates compared with external fixation devices. ä The emerging role of the antibiotic cement-coated interlocking IMN may expand the indications to achieve knee fusion in a single-stage intervention. ä Massive bone defects after failure of an infected TKA can be managed with various surgical strategies in a single- stage intervention to preserve leg length and function. Primary total knee arthroplasty (TKA) is a common procedure suppressive antibiotics for recurrent PJIs are generally reserved with a reported increase of 162% from 1991 to 2010 in the for patients with more severe preoperative disability and United States1,2. From 2005 to 2030, it is projected that the medical comorbidity and those who are not candidates to number of TKA procedures will grow by 673% or 3.5 million. -
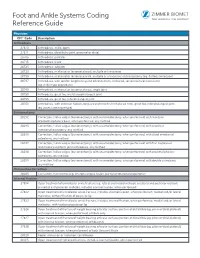
Foot and Ankle Systems Coding Reference Guide
Foot and Ankle Systems Coding Reference Guide Physician CPT® Code Description Arthrodesis 27870 Arthrodesis, ankle, open 27871 Arthrodesis, tibiofibular joint, proximal or distal 28705 Arthrodesis; pantalar 28715 Arthrodesis; triple 28725 Arthrodesis; subtalar 28730 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse 28735 Arthrodesis, midtarsal or tarsometatarsal, multiple or transverse; with osteotomy (eg, flatfoot correction) 28737 Arthrodesis, with tendon lengthening and advancement, midtarsal, tarsal navicular-cuneiform (eg, miller type procedure) 28740 Arthrodesis, midtarsal or tarsometatarsal, single joint 28750 Arthrodesis, great toe; metatarsophalangeal joint 28755 Arthrodesis, great toe; interphalangeal joint 28760 Arthrodesis, with extensor hallucis longus transfer to first metatarsal neck, great toe, interphalangeal joint (eg, jones type procedure) Bunionectomy 28292 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with resection of proximal phalanx base, when performed, any method 28295 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with proximal metatarsal osteotomy, any method 28296 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with distal metatarsal osteotomy, any method 28297 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with first metatarsal and medial cuneiform joint arthrodesis, any method 28298 Correction, hallux valgus (bunionectomy), with sesamoidectomy, when performed; with -

USE of INTERPOSITIONAL BONE GRAFTS for FIRST METARSOPHALANGEAL ARTHRODESIS: a Review of Current Literature
CHAPTER 8 USE OF INTERPOSITIONAL BONE GRAFTS FOR FIRST METARSOPHALANGEAL ARTHRODESIS: A Review of Current Literature Thomas A. Brosky, II, DPM Cayla Conway, DPM INTRODUCTION incidence of nonunion with the use of autogenic iliac crest graft (26.7%) compared to the allograft (0%). Surgical procedures for fi rst metarsophalangeal joint (MPJ) In contrast to the Myerson study, Mankovecky et al arthritis have been well outlined in podiatric literature. (2) suggested a much lower rate of nonunion with the use The fi rst MPJ fusion is a procedure that is commonly autogenous bone graft. In 2013, they reported a review of 6 used for initial treatment of this condition or subsequent studies, which reported a total of 42 procedures of fi rst MPJ treatment, after another surgical procedure has failed. A arthrodesis with autogenous graft as a salvage procedure for failed arthroplasty may result in an excessively shortened a failed Keller arthroplasty. Out of the 42 cases, there were fi rst metatarsal or an overly resected proximal phalanx, 2 reported nonunions for a nonunion rate of 4.8%, which which may necessitate the use of an interpositional bone- is signifi cantly less than the rate reported by Myerson. One block graft to restore adequate length. The preservation of of the nonunions was revised, and the other was reported length can pose a surgical challenge, however, it is integral as asymptomatic. The review states the cases were highly in preventing complications that may result from altered varied in technique and fi xation approach. Mankovecky et al biomechanics, such as inadequate propulsion and lesser reported a need for further research to standardize the data metatarsalgia. -

Knee-Arthrodesis-1.Pdf
The Knee 24 (2017) 91–99 Contents lists available at ScienceDirect The Knee Knee arthrodesis by the Ilizarov method in the treatment of total knee arthroplasty failure Andrea Antonio Maria Bruno a,⁎, Alexander Kirienko b, Andrea Peccati c, Paolo Dupplicato a, Massimo De Donato a, Enrico Arnaldi a, Nicola Portinaro c a Arthroscopic and Reconstructive Surgery of the Knee Unit, Humanitas Research Hospital, Rozzano, Milan, Italy b External Fixation Unit, Humanitas Research Hospital, Rozzano, Milan, Italy c Orthopaedics Department, University of Milan, Milan, Italy article info abstract Article history: Background: Currently, the main indication for knee arthrodesis is septic failure of a total knee Received 8 June 2015 arthroplasty (TKA). The purpose of this study was to evaluate the results of knee arthrodesis by Received in revised form 22 September 2016 circular external fixation performed in the treatment of TKA failure in which revision Accepted 3 November 2016 arthroplasty was not indicated. Methods: The study involved 19 patients who underwent knee arthrodesis by the Ilizarov method. Clinical and functional assessments were performed, including Knee Society Score Keywords: (KSS). A postoperative clinical and radiographic evaluation was conducted every three months TKA failure Ilizarov method until the end of the treatment. Postoperative complications and eventual leg shortening were Knee arthrodesis recorded. Results: KSS results showed a significant improvement with respect to the preoperative condi- tion. Of the 16 patients in the final follow-up, 15 patients (93.7%) achieved complete bone fu- sion. Major complications occurred in patients treated for septic failure of TKA and most occurred in patients over 75 years of age; the mean final leg shortening was four centimeters. -

Technical Aspects of Orthotopic Liver Transplantation for Hepatocellular Carcinoma
Technical Aspects of Orthotopic Liver Transplantation for Hepatocellular Carcinoma a a,b, Lung-Yi Lee, MD , David P. Foley, MD * KEYWORDS Liver transplantation Surgery Hepatocellular carcinoma Piggyback technique Portal vein thrombosis KEY POINTS In the majority of cases, patients with cirrhosis and hepatocellular carcinoma (HCC) who undergo liver transplantation are transplanted based on their higher Model for End-Stage Liver Disease (MELD) exception score and not their physiologic MELD score; this usually results in fewer physiologic derangements during liver transplantation. Patients who have previously undergone locoregional therapy or liver resection for HCC can develop significant perihepatic adhesions that increase the complexity of the hepa- tectomy during transplant. Implantation strategy of the inferior vena cava (IVC) during liver transplant may need to be modified based on location of previously treated HCC. Patients who undergo transarterial chemoembolization for pretransplant HCC therapy may have higher rates of hepatic artery thrombosis after liver transplant; therefore, aorto- hepatic bypass grafting with donor iliac artery may be required for arterial in flow to the liver allograft. Patients with portal vein (PV) thrombosis with a bland thrombus and a patent superior mesenteric vein (SMV) can undergo successful liver transplant through either PV throm- bectomy and standard end-to-end PV-PV anastomosis, or the use of SMV-PV bypass graft with donor iliac vein. a Department of Surgery, University of Wisconsin School of Medicine and Public Health, Clinical Sciences Center, H4/766, 600 Highland Avenue, Madison, WI 53792-3284, USA; b Veterans Administration Surgical Services, William S. Middleton Memorial Veterans Hospital, 2500 Overlook Terrace, Madison, WI 53705, USA * Corresponding author. -

Icd-9-Cm (2010)
ICD-9-CM (2010) PROCEDURE CODE LONG DESCRIPTION SHORT DESCRIPTION 0001 Therapeutic ultrasound of vessels of head and neck Ther ult head & neck ves 0002 Therapeutic ultrasound of heart Ther ultrasound of heart 0003 Therapeutic ultrasound of peripheral vascular vessels Ther ult peripheral ves 0009 Other therapeutic ultrasound Other therapeutic ultsnd 0010 Implantation of chemotherapeutic agent Implant chemothera agent 0011 Infusion of drotrecogin alfa (activated) Infus drotrecogin alfa 0012 Administration of inhaled nitric oxide Adm inhal nitric oxide 0013 Injection or infusion of nesiritide Inject/infus nesiritide 0014 Injection or infusion of oxazolidinone class of antibiotics Injection oxazolidinone 0015 High-dose infusion interleukin-2 [IL-2] High-dose infusion IL-2 0016 Pressurized treatment of venous bypass graft [conduit] with pharmaceutical substance Pressurized treat graft 0017 Infusion of vasopressor agent Infusion of vasopressor 0018 Infusion of immunosuppressive antibody therapy Infus immunosup antibody 0019 Disruption of blood brain barrier via infusion [BBBD] BBBD via infusion 0021 Intravascular imaging of extracranial cerebral vessels IVUS extracran cereb ves 0022 Intravascular imaging of intrathoracic vessels IVUS intrathoracic ves 0023 Intravascular imaging of peripheral vessels IVUS peripheral vessels 0024 Intravascular imaging of coronary vessels IVUS coronary vessels 0025 Intravascular imaging of renal vessels IVUS renal vessels 0028 Intravascular imaging, other specified vessel(s) Intravascul imaging NEC 0029 Intravascular -

Controls on Anastomosis in Lowland River Systems: Towards Process-Based Solutions to 2 Habitat Conservation
1 Controls on anastomosis in lowland river systems: towards process-based solutions to 2 habitat conservation 3 Paweł Marcinkowski1, Robert C. Grabowski2*, Tomasz Okruszko1 4 1Department of Water Engineering, Faculty of Civil and Environmental Engineering, Warsaw University of Life 5 Sciences (WULS-SGGW), Nowoursynowska 159 street, 02-776 Warszawa, Poland, e-mail: 6 [email protected], [email protected] 7 2 School of Water, Energy, and Environment. Cranfield University, College Road, Cranfield, Bedfordshire, MK43 8 0AL, UK. 9 10 * Corresponding author: [email protected] 11 This is the authors’ manuscript. The final article is available from Science of the Total 12 Environment. 13 Abstract 14 Anastomosing rivers were historically common around the world before extensive agricultural and industrial 15 development in river valleys. Few lowland anastomosing rivers remain in temperate zones, and the protection of 16 these river-floodplain systems is an international conservation priority. However, the mechanisms that drive the 17 creation and maintenance of multiple channels, i.e. anabranches, are not well understood, particularly for lowland 18 rivers, making it challenging to identify effective management strategies. This study uses a novel multi-scale, 19 process-based hydro-geomorphological approach to investigate the natural and anthropogenic controls on 20 anastomosis in lowland river reaches. Using a wide range of data (hydrologic, cartographic, remote-sensing, 21 historical), the study (i) quantifies changes in the planform of the River Narew, Poland over the last 100 years, (ii) 22 documents changes in the natural and anthropogenic factors that could be driving the geomorphic change, and (iii) 23 develops a conceptual model of the controls of anastomosis.