Western Research Parks 2017-2018 Annual Report
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
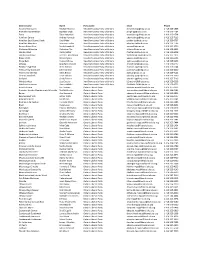
District Name
District name Name Party name Email Phone Algoma-Manitoulin Michael Mantha New Democratic Party of Ontario [email protected] 1 416 325-1938 Bramalea-Gore-Malton Jagmeet Singh New Democratic Party of Ontario [email protected] 1 416 325-1784 Essex Taras Natyshak New Democratic Party of Ontario [email protected] 1 416 325-0714 Hamilton Centre Andrea Horwath New Democratic Party of Ontario [email protected] 1 416 325-7116 Hamilton East-Stoney Creek Paul Miller New Democratic Party of Ontario [email protected] 1 416 325-0707 Hamilton Mountain Monique Taylor New Democratic Party of Ontario [email protected] 1 416 325-1796 Kenora-Rainy River Sarah Campbell New Democratic Party of Ontario [email protected] 1 416 325-2750 Kitchener-Waterloo Catherine Fife New Democratic Party of Ontario [email protected] 1 416 325-6913 London West Peggy Sattler New Democratic Party of Ontario [email protected] 1 416 325-6908 London-Fanshawe Teresa J. Armstrong New Democratic Party of Ontario [email protected] 1 416 325-1872 Niagara Falls Wayne Gates New Democratic Party of Ontario [email protected] 1 416 212-6102 Nickel Belt France GŽlinas New Democratic Party of Ontario [email protected] 1 416 325-9203 Oshawa Jennifer K. French New Democratic Party of Ontario [email protected] 1 416 325-0117 Parkdale-High Park Cheri DiNovo New Democratic Party of Ontario [email protected] 1 416 325-0244 Timiskaming-Cochrane John Vanthof New Democratic Party of Ontario [email protected] 1 416 325-2000 Timmins-James Bay Gilles Bisson -

46581 Ivey Lecture Book
Volume 1, February 2005 Lawrence National Centre for Policy and Management The 1st Annual Lawrence Distinguished Lecture In Policy and Management Strengthening The Canadian Advantage: FOSTERING PRODUCTIVITY THROUGH SOUND PUBLIC POLICY Dr. Kevin Lynch Volume 1, February 2005 The 1st Annual Lawrence Distinguished Lecture In Policy and Management Strengthening The Canadian Advantage: FOSTERING PRODUCTIVITY THROUGH SOUND PUBLIC POLICY Dr. Kevin Lynch Message from the Chair THOMAS P’AQUINOP The Lawrence Centre at the Ivey Business School aspires to be at the cutting-edge of thinking about the nexus between public policy and busi- ness strategy. In February of this year, the Centre hosted the inaugural Lawrence Distinguished Lecture in Policy and Management. Delivered by a distin- guished personality in the service of the Government of Canada, Kevin Lynch, the lecture addresses issues critical to Canadian competitive- ness. Dr. Lynch’s presentation sets a high standard for future lectures that will offer fresh and provocative ideas from leaders in the world of politics, business, academe and the media. The Lawrence lecture series is an important com- ponent of the Centre’s overall mandate: to advance thinking, research and action in the interdependent domains of public policy and business strategy. In sharing Dr. Lynch’s ideas with our readers, we trust that you will be both inspired and motivated to take notice of our work and to support our endeavours. Thomas d’Aquino is a lawyer, entrepreneur, author and strategist. He is Chief Executive and President of the Canadian Council of Chief Executives (CCCE), a non-partisan, not-for-profit organization composed of the chief executives of Canada’s leading 150 enterprises. -

'Turncoats, Opportunists, and Political Whores': Floor Crossers in Ontario
“‘Turncoats, Opportunists, and Political Whores’: Floor Crossers in Ontario Political History” By Patrick DeRochie 2011-12 Intern Ontario Legislature Internship Programme (OLIP) 1303A Whitney Block Queen’s Park Toronto, Ontario M7A 1A2 Phone: 416-325-0040 [email protected] www.olipinterns.ca www.facebook.com/olipinterns www.twitter.com/olipinterns Paper presented at the 2012 Annual meeting of the Canadian Political Science Association Edmonton, Alberta Friday, June 15th, 2012. Draft: DO NOT CITE 2 Acknowledgements I would like to thank the following people for their support, advice and openness in helping me complete this research paper: Gilles Bisson Sean Conway Steve Gilchrist Henry Jacek Sylvia Jones Rosario Marchese Lynn Morrison Graham Murray David Ramsay Greg Sorbara Lise St-Denis David Warner Graham White 3 INTRODUCTION When the October 2011 Ontario general election saw Premier Dalton McGuinty’s Liberals win a “major minority”, there was speculation at Queen’s Park that a Member of Provincial Parliament (MPP) from the Progressive Conservative (PC) Party or New Democratic Party (NDP) would be induced to cross the floor. The Liberals had captured fifty-three of 107 seats; the PCs and NDP, thirty-seven and seventeen, respectively. A Member of one of the opposition parties defecting to join the Liberals would have definitively changed the balance of power in the Legislature. Even with the Speaker coming from the Liberals’ ranks, a floor crossing would give the Liberals a de facto majority and sufficient seats to drive forward their legislative agenda without having to rely on at least one of the opposition parties. A January article in the Toronto Star revealed that the Liberals had quietly made overtures to at least four PC and NDP MPPs since the October election, 1 meaning that a floor crossing was a very real possibility. -

Ivey Idea Forum
PRESENTATION OVERVIEWS 3 PARTICIPANT DISCUSSION 7 AGENDA 12 PRESENTERS 13 LIST OF PARTICIPANTS 14 2 Moderator: DAVID SPARLING, Professor and Chair of Agri-Food Innovation and Regulation, Richard Ivey School of Business Water and Food Innovation Driven by Economics The ability to link energy and water consumption to profits makes good business sense. Within the Ontario Food Processing Sector, differences exist in awareness and ability to adopt sustainable water use practices. The Ontario Food Processing Sector represents: • the 2nd largest manufacturing sector in Ontario; • 3 000 establishments and 114 000 employers; • 68% of firms having fewer than 20 employees; • 2 to 3% profit margin. Increased interest in the sector is resulting from: • high expectations in terms of feeding the global population, in a sustainable manner; • relative economic stability and protection from global recession as compared to other sectors; • continual, incremental improvements in efficiency, competitiveness and productivity. Drivers for efficiency in the Agriculture and Agri-Food industry relate to: • lack of scale and investment in the Canadian Food Processing sector, as compared with other regions; • exports, accounting for a large proportion of production, and adapting to a high Canadian dollar; • increased pressure by customers on suppliers for sustainably produced products. Drivers for innovation and competitiveness identified by the Ontario Food Processing industry include: • increasing market share and the desire to expand into new markets; • driving waste and costs out of the system, particularly in terms of water and energy; • meeting food safety and health regulations. Many companies are forming internal change groups, and different relationships with suppliers, customers, consultants, governments and university researchers, to address sustainability issues. -

2018 Election Liberal Party of Ontario Candidates
2018 Election Liberal Party of Ontario Candidates NAME RIDING WEBSITE LINK Joe Dickson Ajax [email protected] Naheed Yaqubian Aurora-Oak Ridges- [email protected] Richmond Hill Ann Hoggarth Barrie-Innisfil [email protected] Robert Quaiff Bay of Quinte [email protected] Arthur Potts Beaches-East York [email protected] Safdar Hussain Brampton Centre [email protected] Dr. Parminder Singh Brampton East [email protected] Harinder Malhi Brampton North [email protected] Sukhwant Thethi Brampton South [email protected] Vic Dhillon Brampton West [email protected] Ruby Toor Brantford-Brant [email protected] Francesca Dobbyn Bruce-Grey-Owen Sound [email protected] Eleanor McMahon Burlington [email protected] Kathryn McGarry Cambridge [email protected] Theresa Qadri Carleton [email protected] Margaret Schleier Stahl Chatham-Kent-Leamington [email protected] Cristina Martins Davenport [email protected] Michael Coteau Don Valley East [email protected] Shelley Carroll Don Valley North [email protected] Kathleen Wynne Don Valley West [email protected] Bob Gordanier Dufferin-Caledon [email protected] Granville Anderson Durham [email protected] 1 | P a g e NAME RIDING WEBSITE LINK Mike Colle Eglinton-Lawrence [email protected] Carlie Forsythe -

LIST of YOUR MPPS in the PROVINCE of ONTARIO | LISTE DE VOS DÉPUTÉS PROVINCIAUX POUR LA PROVINCE DE L’ONTARIO As of April 2021 | À Jour Du Mois D’Avril 2021
LIST OF YOUR MPPS IN THE PROVINCE OF ONTARIO | LISTE DE VOS DÉPUTÉS PROVINCIAUX POUR LA PROVINCE DE L’ONTARIO As of April 2021 | À jour du mois d’avril 2021 NAME | NOM RIDING | CIRCONSCRIPTION CAUCUS | PARTI Anand, Deepak Mississauga—Malton Progressive Conservative Party of Ontario Andrew, Jill Toronto—St. Paul's New Democratic Party of Ontario Armstrong, Teresa J. London—Fanshawe New Democratic Party of Ontario Arnott, Hon. Ted Wellington—Halton Hills Progressive Conservative Party of Ontario Arthur, Ian Kingston and the Islands New Democratic Party of Ontario Baber, Roman York Centre Independent Babikian, Aris Scarborough—Agincourt Progressive Conservative Party of Ontario Bailey, Robert Sarnia—Lambton Progressive Conservative Party of Ontario Barrett, Toby Haldimand—Norfolk Progressive Conservative Party of Ontario Begum, Doly Scarborough Southwest New Democratic Party of Ontario Bell, Jessica University—Rosedale New Democratic Party of Ontario Berns-McGown, Rima Beaches—East York New Democratic Party of Ontario Bethlenfalvy, Hon. Peter Pickering—Uxbridge Progressive Conservative Party of Ontario Bisson, Gilles Timmins New Democratic Party of Ontario Blais, Stephen Orléans Ontario Liberal Party Bouma, Will Brantford—Brant Progressive Conservative Party of Ontario Bourgouin, Guy Mushkegowuk—James Bay New Democratic Party of Ontario Burch, Jeff Niagara Centre New Democratic Party of Ontario G:\Hotlines\President's Message 2021\2021-04-14_List of MPPS in Ontario.docx Calandra, Hon. Paul Markham—Stouffville Progressive Conservative Party -

The London-Middlesex Town Hall on Electoral Reform FINAL REPORT
The London-Middlesex Town Hall on Electoral Reform FINAL REPORT Date: Sunday, September 25, 2016 Time: 1:00PM to 4:00PM Location: King’s University College at Western University Members of Parliament Participating: Peter Fragiskatos (London North Centre) Irene Mathyssen (London Fanshawe) Karen Vecchio (Elgin-Middlesex-London) Kate Young (London West) 1 On Sunday September 25, 2016, a Town Hall meeting on Electoral Reform was held in London Ontario. Local MPs Peter Fragiskatos (London North Centre), Irene Mathyssen (London Fanshawe), Karen Vecchio (Elgin-Middlesex-London), and Kate Young (London West) were in attendance to hear the views of London- Middlesex citizens on our current electoral system and its potential alternatives. Over 300 participants gathered to take part in the discussion at King’s University College. The Town Hall format was non-partisan, with MPs from each of the three political parties representing London-Middlesex constituencies and an afternoon of dialogue framed by an expert presentation. The Town Hall began with a plenary session moderated by Dr. Paul Nesbitt-Larking, Professor of Political Science and Acting Dean of Huron University College, who outlined the workings of various electoral systems and the complex issues arising in translating citizen votes into legislative representation. Following a Question and Answer period, Town Hall participants proceeded to breakout groups of approximately 20 people to discuss five questions: 1. What do you think about the current system for electing Members of Parliament (benefits/flaws)? Do you feel that votes are fairly translated? 2. Do you have a preferred alternative to the current system? What specific features are important to you in an electoral system (for example local representation, proportionality, simplicity, legitimacy etc.)? 3. -

Master of Arts the University of Western Ontario O Gregory K.R. Stort
The Maintenance of Suburban Autonomy: The Story of the Village of Petersville-London West. Ontario 1874- 1897 by Gregory K. R. Stott Department of History Submitted in partial fulfi!ment of the requirements of the degree of Master of Arts Faculty of Graduate Studies The University of Western Ontario London, Ontario July, 1999 O Gregory K.R. Stort National Library Bibliotheque nationale of Canada du Canada Acquisitions and Acquisitions et Bibliographic Services services bibliographiques 395 Wellington Street 395, rue Wellington Ottawa ON K 1A ON4 Ottawa ON K1A ON4 Canada Canada Your hld Vorre reference Our 610 Nolre rdterence The author has granted a non- L'auteur a accorde une licence non exclusive licence allowing the exclusive pennettant a la National Library of Canada to Bibiiotheque nationale du Canada de reproduce, loan, distribute or sell reproduire, preter, distribuer ou copies of this thesis in microform, vendre des copies de cette thbe sous paper or electronic formats. la fonne de microfiche/filrn, de reproduction sur papier ou sur format eiectronique. The author retains ownershp of the L'auteur conserve la propriete du copyright in this thesis. Neither the droit d'auteur qui protege cette these. thesis nor substantial extracts from it Ni la these ni des extraits substantiels may be printed or otherwise de celle-ci ne doivent itre imprimis reproduced without the author's ou autrernent reproduits sans son permission. autorisation. Abstract While the investigation of nineteenth-century suburbs is a relatively new field in North American social history, the study is particularly neglected in the context of Ontario. Frequendy hstorians and antiquarians have deduced that suburban comrnuniries opted to be annexed by cities in order to tap into the infrastructure and services offered in the urban setting- sewices that were out of reach to the smaller municipal corporations themselves. -

The Thames River Watershed: a Background Study for Nomination Under the Canadian Heritage Rivers System 1 9 9 8
Canadian Heritage Rivers System The Thames River Watershed: A Background Study for Nomination under the Canadian Heritage Rivers System 1 9 9 8 The Canadian Heritage Rivers System T A B L E O F C O N T E N T S i The Thames River Watershed: A Background Study for Nomination under the Canadian Heritage Rivers System 1998 Written by the Thames River Background Study Research Team Published by the Upper Thames River Conservation Authority for the Thames River Coordinating Committee Principal Authors: Ian Wilcox Introduction and Conclusion Cathy Quinlan Natural Heritage Cathy Rogers Human Heritage Michael Troughton Human Heritage, Pre-contact Ian McCallum First Nations Heritage Andrea Quenneville Recreation Eleanor Heagy Editing Don Dool Layout and Graphics Copies of this report may be obtained from: The Upper Thames River Conservation Authority 1424 Clarke Road, London Ont. N5V 5B9 Phone: (519) 451-2800 Fax: (519) 451-1188 E-mail: [email protected] Web Site: http://www.thamesriver.org Copyright © Upper Thames River Conservation Authority 1998 ISBN 1-894329-00-7 T A B L E O F C O N T E N T S i Acknowledgments The Thames River Background Study is the product of a large team of agencies, community groups and individuals. As with any community based project, there is rarely time or space to adequately thank all who have offered their time, finances and writing and editorial skills. In light of this, the Background Studies Subcommittee for the Thames River Nomina- tion extends a blanket thank-you to all who contributed to this project. -

Wed 27 Oct 1999 / Mer 27 Oct 1999
No. 5B No 5B ISSN 1180-2987 Legislative Assembly Assemblée législative of Ontario de l’Ontario First Session, 37th Parliament Première session, 37e législature Official Report Journal of Debates des débats (Hansard) (Hansard) Wednesday 27 October 1999 Mercredi 27 octobre 1999 Speaker Président Honourable Gary Carr L’honorable Gary Carr Clerk Greffier Claude L. DesRosiers Claude L. DesRosiers Hansard on the Internet Le Journal des débats sur Internet Hansard and other documents of the Legislative Assembly L’adresse pour faire paraître sur votre ordinateur personnel can be on your personal computer within hours after each le Journal et d’autres documents de l’Assemblée législative sitting. The address is: en quelques heures seulement après la séance est : http://www.ontla.on.ca/ Index inquiries Renseignements sur l’index Reference to a cumulative index of previous issues may be Adressez vos questions portant sur des numéros précédents obtained by calling the Hansard Reporting Service indexing du Journal des débats au personnel de l’index, qui vous staff at 416-325-7410 or 325-3708. fourniront des références aux pages dans l’index cumulatif, en composant le 416-325-7410 ou le 325-3708. Copies of Hansard Exemplaires du Journal Information regarding purchase of copies of Hansard may Pour des exemplaires, veuillez prendre contact avec be obtained from Publications Ontario, Management Board Publications Ontario, Secrétariat du Conseil de gestion, Secretariat, 50 Grosvenor Street, Toronto, Ontario, M7A 50 rue Grosvenor, Toronto (Ontario) M7A 1N8. Par 1N8. Phone 416-326-5310, 326-5311 or toll-free téléphone : 416-326-5310, 326-5311, ou sans frais : 1-800-668-9938. -

Year in Review
Bill 65, passed on May 10, 2000 during the 37th Session, founded the Ontario Association of Former OAFP Parliamentarians. It was the first bill in Ontario history to be introduced by a Legislative Committee. The Ontario Association of Former Parliamentarians Year in Review - 2015 page 1|InFormer Year In Review 2015 Bill 65, passed on May 10, 2000 during the 37th Session, founded the Ontario Association of Former OAFP Parliamentarians. It was the first bill in Ontario history to be introduced by a Legislative Committee. Our Colleagues Who Passed Away in 2015 They served their constituents, their Party and their Province Eric Gordon Cunningham (April 14, 1949 – January 1, 2015) Liberal, Wentworth North 1975 – 1984 Wayne Wettlaufer (December 16, 1943 – June 21, 2015) Progressive Conservative, Kitchener Centre 1995 – 2003 Keith Brown (November 7, 1926 – July 7, 2015) Progressive Conserva- tive, Peterborough 1959 – 1967 Robert TS Frankford (August 1, 1939 – August 1, 2015) New Demo- crat, Scarborough East 1990-1995 Joan Fawcett (April 19, 1937 – August 16, 2015) Liberal, Northumber- land 1987-1995 Derwyn Shea (September 1, 1937 – August 15, 2015) Progressive Conservative High Park - Swansea 1995-1999 Hugh O’Neil (July 10, 1936 – September 14, 2015) Liberal, Quinte 1975-1995 John Ferris (January 29, 1933 - September 27, 2015) Liberal, London South 1975-1977 William Leo Jordan (December 29, 1929 – February 15, 2015) Progres- sive Conservative, Lanark – Renfrew 1990 - 1999 Year In Review 2015 page 2|InFormer Bill 65, passed on May 10, 2000 during the 37th Session, founded the Ontario Association of Former OAFP Parliamentarians. It was the first bill in Ontario history to be introduced by a Legislative Committee. -

They Build on Great Relationships... It’S in Their DNA
They Build on Great Relationships... It’s in their DNA The Liberal Family Tree The London Connection David Peterson (Premier of Ontario, 1985-1990) First elected in 1975 in London Centre, named Liberal Leader in 1982. Now senior partner with Bay Street law firm Cassels, Brock & Blackwell LLP where he recruited privatizer Mike Harris (Tory premier 1995-2002() as an advisor.. Close with Don Smith, founder of building giant EllisDon, Liberal Party president 1985-87 and chief fundraiser for the Liberals. Peterson credits Smith with helping him restore the Liberal Party after 42 Tory years. Peterson has maintained close ties to the Liberals. He advised Dalton McGuinty throughout the latter’s time as premier (2003-2013) He has served on the boards of Rogers Communications and Shoppers Drug Mart (while it was being acquired by Loblaws). Chair of the Pan Am Games organizing committee. Shelley Peterson Wife of David Peterson and sister of Deb Matthews. Deb Matthews (Deputy Premier and President of the Treasury Board) Party activist since 1975 when she helped run David Peterson’s successful campaign in London Centre. In 1987 and 1995 she co-chaired the Liberal Party’s provincial campaign (her co-chair in the 1987 campaign was David McNaughton). President of the Ontario Liberal Party (2003-2006). Elected for London North Centre in 2003 and held cabinet posts in the McGuinty and Wynne governments. As Minister of Health and Long-Term Care (2009-2014) she presided over the Ornge Air Ambulance scandal. As President of the Treasury Board she is mandated to oversee debt reduction, including reducing the cost of public services and managing public sector compensation.