University Microfilms International300 N
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Endangered, Threatened, Extinct, Endemic, and Rare Or Restricted Utah Vascular Plants
Great Basin Naturalist Volume 35 Number 4 Article 1 12-31-1975 Endangered, threatened, extinct, endemic, and rare or restricted Utah vascular plants Stanley L. Welsh Brigham Young University N. Duane Atwood Bureau of Land Management, Cedar City, Utah James L. Reveal University of Maryland, College Park, and Smithsonian Institution, Washington, D.C. Follow this and additional works at: https://scholarsarchive.byu.edu/gbn Recommended Citation Welsh, Stanley L.; Atwood, N. Duane; and Reveal, James L. (1975) "Endangered, threatened, extinct, endemic, and rare or restricted Utah vascular plants," Great Basin Naturalist: Vol. 35 : No. 4 , Article 1. Available at: https://scholarsarchive.byu.edu/gbn/vol35/iss4/1 This Article is brought to you for free and open access by the Western North American Naturalist Publications at BYU ScholarsArchive. It has been accepted for inclusion in Great Basin Naturalist by an authorized editor of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. The Great Basin Naturalist Published at Provo, Utah, by Brigham Young University Volume 35 December 31, 1975 No. 4 ENDANGERED, THREATENED, EXTINCT, ENDEMIC, AND RARE OR RESTRICTED UTAH VASCULAR PLANTS Stanley L. Welshi, N. Duane Atwood-, and James L. ReveaP Abstract.— The status of 382 vascular plant taxa with distribution in Utah is presented. Some 66 species are possibly endangered, 198 threatened, 7 extinct, and 20 extirpated within the state; 4 spe- cies have questionable taxonomic status. Included in the list are nearly 225 species of endemic plants, many of which are among the possibly endangered, threatened, and extinct or extirpated plants. Bibliographic citations, type locality, status, and distribution by counties is included for each species or infraspecific taxon. -

Lake Havasu City Recommended Landscaping Plant List
Lake Havasu City Recommended Landscaping Plant List Lake Havasu City Recommended Landscaping Plant List Disclaimer Lake Havasu City has revised the recommended landscaping plant list. This new list consists of plants that can be adapted to desert environments in the Southwestern United States. This list only contains water conscious species classified as having very low, low, and low-medium water use requirements. Species that are classified as having medium or higher water use requirements were not permitted on this list. Such water use classification is determined by the type of plant, its average size, and its water requirements compared to other plants. For example, a large tree may be classified as having low water use requirements if it requires a low amount of water compared to most other large trees. This list is not intended to restrict what plants residents choose to plant in their yards, and this list may include plant species that may not survive or prosper in certain desert microclimates such as those with lower elevations or higher temperatures. In addition, this list is not intended to be a list of the only plants allowed in the region, nor is it intended to be an exhaustive list of all desert-appropriate plants capable of surviving in the region. This list was created with the intention to help residents, businesses, and landscapers make informed decisions on which plants to landscape that are water conscious and appropriate for specific environmental conditions. Lake Havasu City does not require the use of any or all plants found on this list. List Characteristics This list is divided between trees, shrubs, groundcovers, vines, succulents and perennials. -
![Nakedstem Sunray (Enceliopsis Nudicaulis [A. Gray] A. Nelson)](https://docslib.b-cdn.net/cover/6874/nakedstem-sunray-enceliopsis-nudicaulis-a-gray-a-nelson-986874.webp)
Nakedstem Sunray (Enceliopsis Nudicaulis [A. Gray] A. Nelson)
NAKEDSTEM SUNRAY Enceliopsis nudicaulis (A. Gray) A. Nelson Asteraceae – Aster family Corey L. Gucker and Nancy L. Shaw | 2020 ORGANIZATION NOMENCLATURE Nakedstem sunray (Enceliopsis nudicaulis) (A. Names, subtaxa, chromosome number(s), hybridization. Gray) A. Nelson belongs to the Ecliptinae subtribe and Heliantheae tribe within the Asteraceae family. Nomenclature follows Welsh et al. (2016). NRCS Plant Code. ENNU (USDA NRCS 2019). Range, habitat, plant associations, elevation, soils. Synonyms. Encelia nudicaulis A. Gray Common Names. Nakedstem sunray, Ash Life form, morphology, distinguishing characteristics, reproduction. Meadows sunray, nakedstem, naked-stemmed daisy, sunray (USFWS 1983; Curtis 2006; Welsh et al. 2016; USDA NRCS 2019). Growth rate, successional status, disturbance ecology, importance to Subtaxa. Some systematists (Cronquist 1972; animals/people. Welsh et al. 2016) recognize nakedstem sunray varieties: corrugata and bairdii, although others do not support varietal distinctions (Sanders and Current or potential uses in restoration. Clark 1987; Curtis 2006). Chromosome Number. Reported chromosome numbers include: 2n = 32, 34, and 36; but most Seed sourcing, wildland seed collection, seed cleaning, storage, consistently reported are 2n = 34 and 36 (Reveal testing and marketing standards. and Styer 1974; Hickman 1993; Curtis 2006; Welsh et al. 2016). Recommendations/guidelines for producing seed. Hybridization. The possibility of hybrids within the Enceliopsis genus have been suggested based on DNA evidence. Sequencing of two nuclear and two chloroplast regions suggests that a single Recommendations/guidelines for producing planting stock. plant collected from co-occurences of nakedstem sunray and Panamint daisy (E. covillei) in California may represent a hybrid or backcrossed individual (Fehlberg and Ranker 2007). Hybrids of nakedstem Recommendations/guidelines, wildland restoration successes/ failures. -

Naomi S. Fraga Rancho Santa Ana Botanic Garden
The California Deserts: Plant Life at the Extremes Naomi S. Fraga Rancho Santa Ana Botanic Garden Red Pass, Death Valley NP Eastern Kern County, California Western Mojave Desert 29 million acres (45,000 sq mi), or 28% of California's landmass. GIS layer source: Omernick ecoregions level 3 Great Basin Mojave Sonoran A Conspiracy of Extremes Bruce Pavlick- 2008 The California Deserts - Topography: 14,246 to -279 ft. - Geology: Limestone, granite, sand dunes - Temperature: below freezing to 134°F (1913) - Changing history over the past 12,000 years - Transition to modern desert complete by 8,500 to 5,000 years ago Krascheninnikovia lanata (winterfat) “Water, water, water....There is no shortage of water in the desert but exactly the right amount, a perfect ratio of water to rock, water to sand, insuring that wide free open, generous spacing among plants and animals, homes and towns and cities, which makes the arid West so different from any other part of the nation. There is no lack of water here unless you try to establish a city where no city should be.” ― Edward Abbey, Desert Solitaire: A Season in the Wilderness Dutch Cleanser Mine, Red Rock Canyon SP Desert Flora - 2377 taxa native to desert (37% of the CA flora) - 785 taxa that do not occur elsewhere in CA (33%) - 232 naturalized taxa (9%) when compared CA (17%) Sources Desert Jepson Manual (2002) Jepson e-flora (2017) Pavlick (2008) Hesperocallis undulata (desert lily) Topographic diversity= species diversity Great Basin 1363 Taxa Mojave 1409 Taxa Sonoran 709 Taxa Remarkable Flora Dr. Frank Vasek’s students circling King Clone on April 1, 1979. -
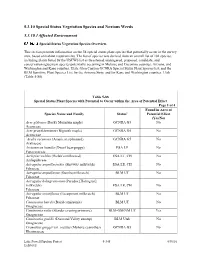
Special Status Vegetation Species and Noxious Weeds
5.3.10 Special Status Vegetation Species and Noxious Weeds 5.3.10.1 Affected Environment Special Status Vegetation Species Overview. This section presents information on the 58 special status plant species that potentially occur in the survey area, based on habitat requirements. The list of species was derived from an overall list of 101 species, including plants listed by the USFWS list as threatened, endangered, proposed, candidate, and conservation agreement species potentially occurring in Mohave and Coconino counties, Arizona, and Washington and Kane counties, Utah; Glen Canyon GCNRA Special Status Plant Species list; and the BLM Sensitive Plant Species List for the Arizona Strip, and for Kane and Washington counties, Utah (Table 5-88). Table 5-88 Special Status Plant Species with Potential to Occur within the Area of Potential Effect Page 1 of 4 Found in Area of Species Name and Family Status1 Potential Effect (Yes/No) Acer glabrum (Rocky Mountain maple) GCNRA G5 No Aceraceae Acer grandidentatum (Bigtooth maple) GCNRA G5 No Aceraceae `Aralia racemosa (American spikenard) GCNRA G5 No Araliaceae Arctomecon humilis (Dwarf bear-poppy) ESA LE No Papaveraceae Asclepias welshii (Welsh’s milkweed) ESA LT, CH No Asclepidaceae Astragalus ampullarioides (Shivwits milkvetch) ESA LE, CH No Fabaceae Astragalus ampullarius (Gumbo milkvetch) BLM UT No Fabaceae Astragalus holmgreniorum (Paradox [Holmgren] milkvetch) ESA LE, CH No Fabaceae Astragalus striatiflorus (Escarpment milkvetch) BLM UT No Fabaceae Camissonia bairdii (Baird camissonia) BLM -

Encelia Farinosa A. Gray Ex Torr NRCS CODE: Tribe: Heliantheae ENFA Family: Asteraceae Order: Asterales Subclass: Asteridae Class: Magnoliopsida
SPECIES Encelia farinosa A. Gray ex Torr NRCS CODE: Tribe: Heliantheae ENFA Family: Asteraceae Order: Asterales Subclass: Asteridae Class: Magnoliopsida juvenile plant, 3/3/2010, Riverside Co. A. Montalvo , 2003-2010, Riverside Co. Subspecific taxa None currently accepted (JepsonOnline 2nd Ed. 2010). Synonyms Encelia farinosa Torry & A. Gray corrected to current authorship (JepsonOnline) ENFAF Encelia farinosa A. Gray ex Torr. var. farinosa brittlebush ENFAP Encelia farinosa A. Gray ex Torr. var. phenicodonta (S.F. Blake) I.M. Johnst. brittlebush ENFAR Encelia farinosa A. Gray ex Torr. var. radians Brandegee ex S.F. Blake Common name brittlebush Also: brittle bush, brittle-bush, brittlebush encelia, incienso, incienso brittlebush, common brittlebush, white brittlebush; brown-center brittlebush for what was considered to be variety phenocodonta ; incienso is used for taxa in multiple families and desert encelia is also used for other species of Encelia (Painter 2009). Taxonomic relationships There are seven other species of Encelia in North America plus additional species in South America (JepsonOnline 2010). The genus Encelia is in the large tribe Heliantheae which also contains the native sunflower, Helianthus annuus L. Phylogenetic studies based on DNA show that Enceliopsis and Geraea are closely related genera and that diversification of species of Encelia has been quite recent (Fehlberg & Ranker 2007). Relationships among the various species of Encelia confirmed hypotheses by Clark (1998) based on chemical and morphological traits. Related taxa in region Four species and spontaneous hybrids of Encelia occur in southern California and may overlap with E. farinosa in some part of its range (FNA 2010, JepsonOnline 2010): E. actoni Elmer (in the southern Sierra Nevada, Tehachapi and Western Transverse Ranges, San Gabriel and San Bernardino Mountains, Mojave and Sonoran Deserts, and Desert Mountains; overlaps with E. -

Bibliography
ASH ME A DOWS REFE R ENCES by Kathleen Capels and Terence Yorks, High Level Research http://www.hlresearch.org/ in cooperation with Frank J. Smith, Western Ecological Services http://ecocorridors.com/western-ecological.php Note: all hyperlinks were checked for validity in late July 2009. Some may require entering the desired genera or local site name into a search space on the linked page to generate appropriate information. Abrams, Leroy, and Roxana Stinchfield Ferris. 1960. Bignoniaceae to Compositae: Begonias to Sunflowers. Vol. 4 of Illustrated Flora of the Pacific States: Washington, Oregon, and California. Stanford, CA: Stanford University Press. [Enceliopsis] Baldwin, Bruce G., Steve Boyd, Barbara J. Ertter, Robert W. Patterson, Thomas J. Rosatti, and Dieter H. Wilken, eds. 2002. The Jepson Desert Manual: Vascular Plants of Southeasten California. Berkeley and Los Angeles: University of California Press. [Centaurium, Ivesia, Nitrophila] Barneby, R[upert] C. 1970 [published 1971]. A new Astragalus (Fabaceae) from Nevada. Madroño 20(8):395–398. [Astragalus] Beacham, Walter, Frank V. Castronova, and Suzanne Sessine. 2000. Dicots. Vol. 4 of Beacham’s Guide to the Endangered Species of North America. Detroit: Gale Group. [Nitrophila] Beatley, Janice C. 1969. Vascular Plants of the Nevada Test Site, Nellis Air Force Range, and Ash Meadows (Northern Mojave and Southern Great Basin Deserts, South-Central Nevada). Los Angeles: University of California Laboratory of Nuclear Medicine and Radiation Biology. [Astragalus, Centaurium, Cordylanthus, Enceliopsis, Grindelia, Ivesia, Mentzelia, Nitrophila, Spiranthes] ———. 1970. Additions to Vascular Plants of the Nevada Test Site, Nellis Air Force Range, and Ash Meadows. Los Angeles: University of California Laboratory of Nuclear Medicine and Radiation Biology. -

Desert Plants of Utah
DESERT PLANTS OF UTAH Original booklet and drawings by Berniece A. Andersen Revised May 1996 HG 505 FOREWORD The original Desert Plants of Utah by Berniece A. Andersen has been a remarkably popular book, serving as a tribute to both her botanical knowledge of the region and to her enthusiastic manner. For these reasons, we have tried to retain as much of the original work, in both content and style, as possible. Some modifications were necessary. We have updated scientific names in accordance with changes that have occurred since the time of the first publication and we have also incorporated new geographic distributional data that have accrued with additional years of botanical exploration. The most obvious difference pertains to the organization of species. In the original version, species were organized phylogenetically, reflecting the predominant concepts of evolutionary relationships among plant families at that time. In an effort to make this version more user-friendly for the beginner, we have chosen to arrange the plants primarily by flower color. We hope that these changes will not diminish the enjoyment gained by anyone familiar with the original. We would also like to thank Larry A. Rupp, Extension Horticulture Specialist, for critical review of the draft and for the cover photo. Linda Allen, Assistant Curator, Intermountain Herbarium Donna H. Falkenborg, Extension Editor TABLE OF CONTENTS The Nature of Deserts ........................................................1 Utah’s Deserts ........................................................2 -

Utah's Mojave Desert Flora
Sego Lily March 2010 33 (2) March 2010 (volume 33 number 2) Utah’s Mojave Desert Flora By Walter Fertig Above: Joshua trees, chollas, creo- snow. Lack of moisture for much sote bush, bur-sage, and other Mo- of the year helps explain the jave Desert shrubs on the flanks of It is no accident that the ―deserted‖ appearance of deserts, the Beaver Dam Mountains. Photo words ―desert‖ and ―deserted‖ share which, with few exceptions, have a by Douglas N. Reynolds. the same origin. Both refer to areas very sparse covering of plants. that seem desolate and empty. High rates of evaporation and cover. High temperatures alone, Ecologists define deserts more pre- transpiration are facilitated by however, do not define deserts, as cisely to include those lands where high temperatures, and not sur- some of the driest areas on Earth are annual rates of evaporation and prisingly most deserts fall within the cold polar deserts of the high transpiration of water by plants ex- latitudes of high solar radiation, arctic and dry valleys of Antarctica. ceeds precipitation from rain and low humidity, and minimal cloud For our purposes, [continued pg 6] Copyright 2010 Utah Native Plant Society. All Rights Reserved. Utah Native Plant Society Education: Ty Harrison Sego Lily Editor: Walter Fertig Horticulture: Maggie Wolf ([email protected]). The deadline for Invasive Weeds: Susan Fitts the May 2010 Sego Lily is 15 April Rare Plants: Walter Fertig 2010. Scholarship: Bill Gray Copyright 2010 Utah Native Plant So- Chapters and Chapter Presidents ciety. All Rights Reserved Cache: Amy Croft and Michael Piep Cedar City: Marguerite Smith The Sego Lily is a publication of the Officers Escalante: Harriet Priska Utah Native Plant Society, a 501(c)(3) President: Walter Fertig (Kane Co) Fremont: Lisa White not-for-profit organization dedicated Vice President: Kipp Lee (Salt Lake Co) Manzanita: Walter Fertig to conserving and promoting steward- Treasurer: Charlene Homan (Salt Lake Mountain: Mindy Wheeler ship of our native plants. -

Annotated Checklist of Vascular Flora, Arches National Park
National Park Service U.S. Department of the Interior Natural Resource Program Center Annotated Checklist of Vascular Flora Arches National Park Natural Resource Technical Report NPS/NCPN/NRTR—2009/220 ON THE COVER Double Arch, Arches National Park, Utah. Photograph by Walter Fertig. Annotated Checklist of Vascular Flora Arches National Park Natural Resource Technical Report NPS/NCPN/NRTR—2009/220 Authors Walter Fertig Moenave Botanical Consulting 1117 W. Grand Canyon Dr. Kanab, UT 84741 Sarah Topp Northern Colorado Plateau Network National Park Service P.O. Box 848 Moab, UT 84532 Mary Moran Southeast Utah Group National Park Service P.O. Box 907 Moab, UT 84532 Editing and Design Alice Wondrak Biel Northern Colorado Plateau Network National Park Service P.O. Box 848 Moab, UT 84532 June 2009 U.S. Department of the Interior National Park Service Natural Resource Program Center Fort Collins, Colorado The National Park Service, Natural Resource Program Center publishes a range of reports that address natural resource topics of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, con- servation and environmental constituencies, and the public. The Natural Resource Technical Report Series is used to disseminate results of scientifi c stud- ies in the physical, biological, and social sciences for both the advancement of science and the achievement of the National Park Service mission. The series provides contributors with a forum for displaying comprehensive data that are often deleted from journals because of page limitations. All manuscripts in the series receive the appropriate level of peer review to ensure that the in- formation is scientifi cally credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Mohave Palisades
Proposed Lands with Wilderness Characteristics: Black Mountains North A proposal report to the Bureau of Land Management, Kingman Field Office, Arizona August, 2015 Prepared by: Joseph M. Trudeau & Amber R. Fields Black Mountains North Proposed LWC Table of Contents PREFACE: This Proposal was developed according to BLM Manual 6310 page 3 MAP: Black Mountains North Proposed Lands with Wilderness Characteristics (LWC) page 5 SECTION 1: Proposed LWC Overview Unit Location page 6 Brief Boundary Description page 6 Landforms & Biological Communities page 6 Previous Wilderness Inventories page 7 SECTION 2: Wilderness Characteristics The proposed LWC meets the minimum size criteria for roadless lands page 7 The proposed LWC is affected primarily by the forces of nature page 7 The proposed LWC provides outstanding opportunities for solitude or primitive & unconfined recreation page 9 The proposed LWC has supplemental values that enhance the wilderness experience & deserve protection page 10 Works Cited page 12 SECTION 3: Detailed Boundary & Routes Description Narrative Description of the Proposed LWC Boundary page 13 SECTION 4: Photopoint data Data Tables & Photographs to accompany the Detailed Boundary & Routes Description page 16 Cover Photo: From the eastern boundary of the proposed LWC, looking across the unit to the southwest, towards Lake Mohave and distant mountains in Nevada. Arizona Wilderness Coalition Page 2 of 22 Trudeau & Fields, 2015 Black Mountains North Proposed LWC PREFACE: This Proposal was developed according to BLM Manual 6310 -
Detrital Wash Watershed – Arizona Rapid Watershed Assessment April 2007
Detrital Wash Watershed – Arizona Rapid Watershed Assessment April 2007 Prepared by: USDA Natural Resource Conservation Service – Arizona University of Arizona, Water Resources Research Center In cooperation with: Arizona Association of Conservation Districts Arizona Department of Agriculture Arizona Department of Environmental Quality Arizona Department of Water Resources Arizona Game & Fish Department Arizona State Land Department USDA Forest Service USDI Bureau of Land Management Released by: Sharon Megdal David McKay Director State Conservationist University of Arizona U.S. Department of Agriculture Water Resources Research Center Natural Resources Conservation Service Principle Investigators: Dino DeSimone – Natural Resources Conservation Service, Phoenix, Arizona Keith Larson – Natural Resources Conservation Service, Phoenix, Arizona Kristine Uhlman – Water Resources Research Center, University of Arizona D. Phil Guertin – School of Natural Resources, University of Arizona Deborah Young – Associate Director, Cooperative Extension, University of Arizona The United States Department of Agriculture (USDA) prohibits discrimination in all its programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at