'Ofe Akwu' Soup Spoilage
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Show Activity
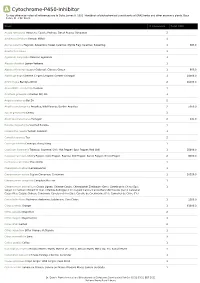
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Seasonal Incidence of Ocimum Tingid Bug, Cochlochila Bullita Stal
Journal of Pharmacognosy and Phytochemistry 2020; 9(4): 3138-3144 E-ISSN: 2278-4136 P-ISSN: 2349-8234 www.phytojournal.com Seasonal incidence of Ocimum tingid bug, JPP 2020; 9(4): 3138-3144 Received: 07-05-2020 Cochlochila bullita Stal (Heteroptera: Tingidae) Accepted: 09-06-2020 on three different species of Ocimum viz. Ocimum Vishav Prakash Rahul basilicum L., Ocimum sanctum L. and Ocimum CSIR-Indian Institute of Integrative Medicine, Canal kilimandscharicum Guerke Road, Jammu, Jammu and Kashmir, India Vishav Prakash Rahul, Bhumika Kapoor, Sougata Sarkar and Sabha Jeet Bhumika Kapoor Project Assistant, CSIR-Indian Institute of Integrative DOI: https://doi.org/10.22271/phyto.2020.v9.i4ae.12093 Medicine, Canal Road, Jammu, Jammu and Kashmir, India Abstract The field experiment was conducted on the seasonal incidence Ocimum lace bug on three different Sougata Sarkar species of Ocimum viz. Ocimum basilicum L., Ocimum sanctum L. and Ocimum kilimandscharicum Research Associate, Scientist, Guerke at CSIR- IIIM, Chatha Farm, Jammu. The data on seasonal fluctuations of Ocimum tingid bug, CSIR-Indian Institute of Cochlochila bullita on various species of Ocimum such as Ocimum basilicum L., Ocimum sanctum L. Integrative Medicine, Canal and Ocimum kilimandscharicum Guerke were first observed during 33rd standard week of August i.e. Road, Jammu, Jammu and 0.40 Mean insect/ plant, 0.2 mean insect/plant and 0.20 mean insect/plant, respectively. The maximum Kashmir, India lace bug population was recorded on sweet basil during 39th standard week i.e. 52.60 mean insect/ plant when weekly mean maximum temperature 29.7 oC, minimum temperature 23.1 oC, morning relative Sabha Jeet Scientist, CSIR-Indian Institute humidity 93.1% and evening relative humidity 75.90 %, rainfall 93.40 mm, and wind speed 2.00 km/hr, of Integrative Medicine, Canal respectively. -

Honey Bee Suite © Rusty Burlew 2015 Master Plant List by Scientific Name United States
Honey Bee Suite Master Plant List by Scientific Name United States © Rusty Burlew 2015 Scientific name Common Name Type of plant Zone Full Link for more information Abelia grandiflora Glossy abelia Shrub 6-9 http://plants.ces.ncsu.edu/plants/all/abelia-x-grandiflora/ Acacia Acacia Thorntree Tree 3-8 http://www.2020site.org/trees/acacia.html Acer circinatum Vine maple Tree 7-8 http://www.nwplants.com/business/catalog/ace_cir.html Acer macrophyllum Bigleaf maple Tree 5-9 http://treesandshrubs.about.com/od/commontrees/p/Big-Leaf-Maple-Acer-macrophyllum.htm Acer negundo L. Box elder Tree 2-10 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=a841 Acer rubrum Red maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer rubrum Swamp maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer saccharinum Silver maple Tree 3-9 http://en.wikipedia.org/wiki/Acer_saccharinum Acer spp. Maple Tree 3-8 http://en.wikipedia.org/wiki/Maple Achillea millefolium Yarrow Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b282 Aesclepias tuberosa Butterfly weed Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b490 Aesculus glabra Buckeye Tree 3-7 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281045&isprofile=1&basic=buckeye -

Herb List Gardens
NORTH HAVEN Herb List Gardens Common Name Botanical Name UsesCat Light Color Height Soil Symbolism ALOE VERA Aloe barbadensis M TT SUNOrangeWD12"-18" Healing ANISE Pimpinella anisum CMTA S/PSH White12"-18" MWD APPLE MINT Mentha suaveolens CFr MTP S/PSHWhite12"-18" MWD Virtue APPLE MINT Mentha rotundifolia S/PSH Mauve4"-6" M ARUGULA Eruca vesicara CM A SUNCream18"-24" MWD Enthusiasm ARUGULA, DWARF Diplotaxis erucoides C A SUNYellow10"-12" WD Straightforward BASIL, AFRICAN BLUE Ocimum kilimandscharicum CFr O A SUNPurple24"-36" M Affection BASIL, AROMA 2 Ocimum basilicum CFr Fl M O A S/PSHWhite18"-24" WD Good Luck BASIL,' AUSSIE SWEETIE' Ocimum basilicum CFr A SUN18"-24" WD Good Wishes BASIL, BOXWOOD Ocimum basilicum CFr Fl O A S/PSH White8"-10" WD BASIL, CINNAMON Ocimum basilicum CFr A SUNLavender 18"-24" MWD Good Wishes BASIL, 'CITRIODORUM' LEMON Ocimum basilicum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, DARK OPAL Ocimum basilicum COA SUNPurple18"-24" MWD Good Wishes BASIL, 'GENOVESE' Ocimum basilicum CFr Fl A S/PSHWhite18"-24" MWD Good Wishes BASIL, 'GREEK COLUMNAR' OR 'AU Ocimum xcitriodorum 'Lesbos' CFr MO A S/PSH 24"-36" MWD BASIL, HOLY Ocimum sanctum Fr Fl A S/PSHWhite or Laven18"-24" MWD Good Luck BASIL, LETTUCE LEAF Ocimum basilicum COA SUNWhite18"-24" MWD Good Wishes BASIL, LIME Ocimum americanum CFr A SUNWhite18"-24" MWD Good Wishes BASIL, 'MAGICAL MICHAEL' Ocimum basilicum CFr Fl O A S/PSHWhite18"-24" WD Good Wishes BASIL, 'MINETTE' Ocimum basilicum CFr O A SUNWhite12"-18" MWD Good Wishes BASIL, MINI PURPLE Ocimum basilicum -

Plant List 2021-08-25 (12:18)
Plant List 2021-09-24 (14:25) Plant Plant Name Botanical Name in Price Stock Per Unit AFRICAN DREAM ROOT - 1 Silene capensis Yes R92 AFRICAN DREAM ROOT - 2 Silene undulata Yes R92 AFRICAN POTATO Hypoxis hemerocallidea Yes R89 AFRICAN POTATO - SILVER-LEAFED STAR FLOWER Hypoxis rigidula Yes R89 AGASTACHE - GOLDEN JUBILEE Agastache foeniculum No R52 AGASTACHE - HYSSOP, WRINKLED GIANT HYSSOP Agastache rugosa Yes R59 AGASTACHE - LICORICE MINT HYSSOP Agastache rupestris No R59 AGASTACHE - PINK POP Agastache astromontana No R54 AGRIMONY Agrimonia eupatoria No R54 AJWAIN Trachyspermum ammi No R49 ALFALFA Medicago sativa Yes R59 ALOE VERA - ORANGE FLOWER A. barbadensis Yes R59 ALOE VERA - YELLOW FLOWER syn A. barbadensis 'Miller' No R59 AMARANTH - ‘LOVE-LIES-BLEEDING’ Amaranthus caudatus No R49 AMARANTH - CHINESE SPINACH Amaranthus species No R49 AMARANTH - GOLDEN GIANT Amaranthus cruentas No R49 AMARANTH - RED LEAF Amaranthus cruentas No R49 ARTICHOKE - GREEN GLOBE Cynara scolymus Yes R54 ARTICHOKE - JERUSALEM Helianthus tuberosus Yes R64 ARTICHOKE - PURPLE GLOBE Cynara scolymus No R54 ASHWAGANDA, INDIAN GINSENG Withania somniferia Yes R59 ASPARAGUS - GARDEN Asparagus officinalis Yes R54 BALLOON FLOWER - PURPLE Platycodon grandiflorus 'Apoyama' Yes R59 BALLOON FLOWER - WHITE Platycodon grandiflorus var. Albus No R59 BASIL - CAMPHOR Ocimum kilimandscharicum Yes R59 BASIL HOLY - GREEN TULSI, RAM TULSI Ocimum Sanctum Yes R54 BASIL HOLY - TULSI KAPOOR Ocimum sanctum Linn. No R54 BASIL HOLY - TULSI TEMPERATE Ocimum africanum No R54 BASIL HOLY - TULSI -
Show Activity
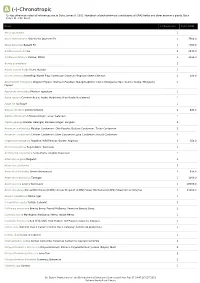
A (-)-Chronotropic *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Abies spectabilis 1 Abies sachalinensis Shin-Yo-Yu; Japanese Fir 1 7560.0 Abies balsamea Balsam Fir 1 4090.0 Achillea moschata Iva 2 4536.0 Achillea millefolium Yarrow; Milfoil 3 3190.0 Acinos suaveolens 2 Acinos alpinus Te de Sierra Nevada 1 Acorus calamus Sweetflag; Myrtle Flag; Sweetroot; Calamus; Flagroot; Sweet Calamus 2 200.0 Aframomum melegueta Alligator Pepper; Grains-of-Paradise; Malagettapfeffer (Ger.); Malagueta (Sp.); Guinea Grains; Melegueta 1 Pepper Ageratum conyzoides Mexican ageratum 1 Ajuga reptans Common Bugle; Bugle; Bugleherb; Blue Bugle; Bugleweed 1 Ajuga iva Ivy Bugle 1 Aloysia citrodora Lemon Verbena 2 840.0 Alpinia officinarum Chinese Ginger; Lesser Galangal 1 Alpinia galanga Greater Galangal; Siamese Ginger; Languas 3 Amomum xanthioides Malabar Cardamom; Chin Kousha; Bastard Cardamom; Tavoy Cardamom 2 Amomum compactum Chester Cardamom; Siam Cardamom; Java Cardamom; Round Cardamom 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 150.0 Annona squamosa Sugar-Apple; Sweetsop 1 Aristolochia serpentaria Serpentaria; Virginia Snakeroot 1 Artemisia vulgaris Mugwort 2 Artemisia salsoloides 3 Artemisia herba-alba Desert Wormwood 3 638.0 Artemisia dracunculus Tarragon 1 1000.0 Artemisia cina Levant Wormseed 1 48000.0 Artemisia annua Annual Wormwood (GRIN); Annual Mugwort (GRIN); Sweet Wormwood -

Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat
Dr. Duke's Phytochemical and Ethnobotanical Databases List of Plants for Candidistat Plants with Activity Synergy Chemical Count Total PPM Abies alba 1 2394.0 Abies balsamea 1 3100.0 Abies spectabilis 2 Acacia farnesiana 1 Achillea millefolium 2 340.0 Acinos suaveolens 3 Acorus calamus 3 27820.0 Aeolanthus myriantha 1 Aesculus hippocastanum 2 Agastache foeniculum 1 Agastache nepetoides 1 Agastache rugosa 1 Agastache urticifolia 1 4928.0 Ageratum conyzoides 1 Allium sativum var. sativum 2 Aloysia citrodora 2 2100.0 Alpinia galanga 2 Alpinia officinarum 2 Amomum compactum 2 Anacardium occidentale 1 Ananas comosus 1 Anethum graveolens 4 Angelica archangelica 3 2640.0 Annona squamosa 1 Apium graveolens 3 1438152.0 Armoracia rusticana 1 Artemisia annua 4 Plants with Activity Synergy Chemical Count Total PPM Artemisia dracunculus 3 Artemisia salsoloides 1 Artemisia vulgaris 1 Asarum canadense 1 Asiasarum sieboldii 1 Asimina triloba 1 1.096 Avena sativa 1 Ballota nigra 1 Boswellia sacra 1 Brassica oleracea var. capitata l. 1 Bursera delpechiana 1 720.0 Calamintha nepeta 1 Callicarpa americana 2 Camellia sinensis 2 Cananga odorata 1 Canarium indicum 1 1000.0 Capsicum annuum 2 Capsicum frutescens 2 Carica papaya 1 Carthamus tinctorius 1 0.04 Carum carvi 3 156060.0 Centella asiatica 2 Chamaemelum nobile 1 Chenopodium ambrosioides 1 Chrysanthemum balsamita 1 Chrysanthemum parthenium 1 Chrysanthemum x morifolium 1 2 Plants with Activity Synergy Chemical Count Total PPM Cinnamomum aromaticum 3 Cinnamomum camphora 1 240.0 Cinnamomum verum 3 384.0 Cistus -

Micropropagation of Ocimum Kilimandscharicum Guerke (Labiatae)
ACTA BIOLOGICA CRACOVIENSIA Series Botanica 52/2: 50–58, 2010 DOI: 10.2478/v10182-010-0023-7 MICROPROPAGATION OF OCIMUM KILIMANDSCHARICUM GUERKE (LABIATAE) SOUMEN SAHA, TULSI DEY, AND PARTHADEB GHOSH* Cytogenetics and Plant Biotechnology Research Unit, Department of Botany, University of Kalyani, Kalyani-741235, West Bengal, India Received March 13, 2010; revision accepted November 3, 2010 An efficient plant regeneration protocol has been developed from nodal explants of Ocimum kilimandscharicum Guerke, a medicinally important herbaceous plant species belonging to the family Lamiaceae. Axillary shoot bud proliferation was initiated from nodal explants cultured on MS medium supplemented with various concentra- tions of 6-benzyladenine (BA) (0.5–3.0 mg/l), kinetin (KN) (0.5–3.0 mg/l) and 2-isoPentenyladenine (2-iP) (0.5–3.0 mg/l). The maximum number of shoots (6.09±0.05), with average length 3.83±0.11 cm, was achieved with medi- um containing 1.0 mg/l BA. Shoot culture was established by repeated subculturing of the original nodal explants on shoot multiplication medium after each harvest of newly formed shoots. In this way, 20–30 shoots were obtained from a single nodal explant after 5 months. Rooting of shoots was achieved on half-strength MS medi- um supplemented with 1.5 mg/1 Indole-3-butyric acid (IBA) and 2% sucrose. Well-developed plantlets transferred to plastic pots containing soil and vermiculite (1:1) showed 81.13% survival. The genetic fidelity of in-vitro-raised field-grown plants to the donor plant was ascertained from random amplified polymorphic DNA (RAPD) markers. -

Eco Friendly Flora
Eco Friendly Flora Parandwadi,Somatane Phata, NH4, Tal-Maval, Dist-Pune 410 506 Contact - 9225104384 / 9422224384 / 8390257511 / 7875695036 Email - [email protected] / [email protected] | Website - www.efshub.com Sr No Common Name Botanical Name Habit Variety Category Aromatic 1 Bhim Kappor Clausena heptaphylla Herb A 2 Chandan Santalum album Tree A 3 Citronela Cymbopogon sp Herb A 4 Dhup (Black Dammer) Canarium strictum Tree A 5 Dhup (RAL) Shorea robusto Tree A 6Dhup uad Vateria indica Tree A 7 Dongri dauna Artemisia pallens Shrub A 8 Lemon grass / Gavti chaha Cymbopogon citratum Grass A 9 Hirwa chafa Artabotrys odoratissimus Shrub A 10 Kapoor Cinnamomum camphora Tree A 11 Kapur Tulas Ocimum kilimandscharicum Herb A 12 Kavthi chafa white Magnolia coco Shrub A 13 Kavthi chafa Yellow Magnolia liliifera Tree A 14 Magnolia (kavthi chafa) Magnolia grandiflora Tree A 15 Kewada Pandanus odorrattissimus Shrub A 16 Krushnagaru Aquilaria agallocha Tree A 17 Lemon bamm Melissa officinalis Herb A 18Lodhra Symplocos racemosa Tree A 19Marwa origanum majorana Herb A 20 Mini Badam Terminalia metalica tree A 21 Nilgiri sented Eucalyptus citriodora Tree A 22 Odomas Pelargonium 'citrosum' Herb A 23Pan Kapur Piper betel Shrub A 24 Ratrani Cestrum nocturnum Shrub A 25 Rosewood Dalbergia latifolia Tree A 26 Rosha grass Cymbopogon sp. Grass A 27 Satap Ruta chalapensis Herb A 28 Sonchapha (Saundaraya) Michelia champaca Tree A 29 Sontakka White Hedychium coronarium Grass A 30 Sontakka Yellow Hedychium flavescens Grass A 31 Vanila Vanilla planifolia Climber -

Ethnoknowledge of Bukusu Community on Livestock Tick Prevention and Control
Journal of Ethnopharmacology 140 (2012) 298–324 Contents lists available at SciVerse ScienceDirect Journal of Ethnopharmacology jo urnal homepage: www.elsevier.com/locate/jethpharm Ethnoknowledge of Bukusu community on livestock tick prevention and control in Bungoma district, western Kenya a,b,∗ c d e b,f Wycliffe Wanzala , Willem Takken , Wolfgang R. Mukabana , Achola O. Pala , Ahmed Hassanali a School of Pure and Applied Sciences, Department of Biological Sciences, South Eastern University College (A Constituent College of the University of Nairobi), P.O. Box 170-90200, Kitui, Kenya b Behavioural and Chemical Ecology Department (BCED), International Centre of Insect Physiology and Ecology (ICIPE), P.O. Box 30772-00100 GPO, Kasarani, Nairobi, Kenya c Wageningen University and Research Centre, Laboratory of Entomology, P.O. Box 8031, 6700 EH Wageningen, The Netherlands d School of Biological Sciences, University of Nairobi, P.O. Box 30197-00100, Nairobi, Kenya e Technology Transfer Unit, International Centre of Insect Physiology and Ecology (ICIPE), P.O. Box, 30772-00100 GPO, Kasarani, Nairobi, Kenya f School of Pure and Applied Sciences, Kenyatta University, P.O. Box 43844-00100 GPO, Nairobi, Kenya a r t i c l e i n f o a b s t r a c t Article history: Ethnopharmacological relevance: To date, nomadic communities in Africa have been the primary focus Received 24 August 2011 of ethnoveterinary research. The Bukusu of western Kenya have an interesting history, with nomadic Received in revised form lifestyle in the past before settling down to either arable or mixed arable/pastoral farming systems. 14 December 2011 Their collective and accumulative ethnoveterinary knowledge is likely to be just as rich and worth Accepted 13 January 2012 documenting. -

Pharmacognostical and Biochemical Investigation of Ocimum Kilimandscharicum Plants Available in Western Himalayan Region
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Asian Journal of Plant Science and Research, 2012, 2 (4):446-451 ISSN : 2249-7412 CODEN (USA): AJPSKY Pharmacognostical and biochemical investigation of Ocimum kilimandscharicum plants available in western Himalayan region Devesh Tewari* a, H. K. Pandey b, A. N. Sah a, H. S. Meena b and A. Manchanda b a Department of Pharmaceutical Sciences Kumaun University Bhimtal Campus Nainital, Uttarakhand, India bDefence Institute of Bio-Energy Research (DIBER), DRDO, Field Station, Pithoragarh, (Uttarakhand) India _____________________________________________________________________________________________ ABSTRACT India has a rich heritage of plants as medicines; Indian systems of medicines utilize 80 percent of the material derived from the plants. Himalaya are a rich repository of tradition, culture and heritage. The diversified topography, soil and microclimatic zones of Himalayan regions have resulted occurrence of several valuable and economically important medicinal and aromatic plants of great therapeutic value. The use of plants as sources of medicines are human substance has been in vogue since antiquity. Large numbers of plants are utilized in various systems of medicine practiced in India and local health traditions for the treatment of human diseases since time immemorial. Ocimum kilimandscharicum Gurke. is used for thousands of years in Ayurveda for its diverse healing properties. Tulsi is the legendary ‘Incomparable one’ of India, is one of the holiest and most cherished of the many healing and healthy giving herbs of the orient. The paper comprises to determine the pharmacognostical parameters and elemental evaluation along with nutritional components and preliminary phytochemical investigation of the Ocimum kilimandscharicum available in western Himalayan region. -

Black Cohosh Adulteration Herbs for Female US/CAN $6.95
HerbalGram 98 • May - July 2013 2013 July - May • 98 HerbalGram Holy Basil Profile • Garlic and Blood Pressure • 100+ Year-Old Phytomedicine Company Artichoke and Cholesterol • ABC Celebration • Chocolate and Stroke Risk Holy Basil Profile • Garlic and Blood Pressure • Herbs Reproductive for Female Health Company Phytomedicine • • Year-Old Herbal 100+ Insect Repellents • Chocolate and Stroke Risk The Journal of the American Botanical Council Number 98 | May - July 2013 Black Cohosh Adulteration Herbs for Female US/CAN $6.95 Reproductive Health www.herbalgram.org www.herbalgram.org Herbal Insect Repellents Herb Profile Holy Basil Ocimum tenuiflorum (syn. O. sanctum) Family: Lamiaceae (formerly Labiatae) INTRODUCTION or “pleasing basil”), is known as Forest or Vana Tulsi. Even Holy basil is a perennial or annual in the mint family though it is a different species, O. gratissimum also is consid- ered sacred in India and is used in the same ways as the O. that exhibits the square stem and volatile oils characteristic 2 of its family.1 It is erect, very branched, strongly aromatic, tenuiflorum varieties. and mildly hairy.2 Holy basil is native to India and parts of Tulsi is one of the principal herbs used in the Ayurvedic M I S S I O N D R I V E N : northern and eastern Africa, Hainan Island, and Taiwan, medicine system, in which it is known alternately as “The Queen of Herbs,” “The Incomparable One,” and “The and grows wild throughout India and up to an altitude of 9 5,900 feet (1,800 meters) in the Himalayas.3-5 In China, Mother Medicine of Nature.” It holds a supreme place in it occurs in dry, sandy areas of Hainan and Sichuan, as the ancient Vedic scriptures and is integrated into daily life well as in Cambodia, Indonesia, Laos, Malaysia, Myan- by Hindus through religious worship.