The Volatile Phytochemistry of Monarda Species Growing in South Alabama
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Electrophysiological and Behavioral Characterization Of
Deletre et al. Parasites & Vectors (2015) 8:316 DOI 10.1186/s13071-015-0934-y RESEARCH Open Access Electrophysiological and behavioral characterization of bioactive compounds of the Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum and Cinnamomum zeylanicum essential oils against Anopheles gambiae and prospects for their use as bednet treatments Emilie Deletre1* , Fabrice Chandre2, Livy Williams3, Claire Duménil1, Chantal Menut4 and Thibaud Martin1,5 Abstract Background: Laboratory and field studies showed that repellent, irritant and toxic actions of common public health insecticides reduce human-vector contact and thereby interrupt disease transmission. One of the more effective strategies to reduce disease risk involves the use of long-lasting treated bednets. However, development of insecticide resistance in mosquito populations makes it imperative to find alternatives to these insecticides. Our previous study identified four essential oils as alternatives to pyrethroids: Thymus vulgaris, Cymbopogon winterianus, Cuminum cyminum, Cinnamomum zeylanicum. The objectives of this study were to identify active compounds of these essential oils, to characterize their biological activity, and to examine their potential as a treatment for bednets. Methods: We evaluated the electrophysiological, behavioural (repellency, irritancy) and toxic effects of the major compounds of these oils against Anopheles gambiae strain ‘Kisumu’. Results: Aldehydes elicited the strongest responses and monoterpenes the weakest responses in electroantennogram (EAG) trials. However, EAG responses did not correlate consistently with results of behavioral assays. In behavioral and toxicity studies, several of the single compounds did exhibit repellency, irritancy or toxicity in An. gambiae; however, the activity of essential oils did not always correlate with activity expected from the major components. On the contrary, the biological activity of essential oils appeared complex, suggesting interactions between individual compounds and the insect under study. -

2009 Wisconsin – Illinois Germplasm Reconnaissance and Collection Trips
NCRPIS 2009 Wisconsin – Illinois Germplasm Reconnaissance and Collection Trips August 3 – 8, 2009 September 21 – 29, 2009 North Central Regional Plant Introduction Station – Ames, IA Wisconsin Germplasm Sites Sauk County Washington County Cornus rugosa Fraxinus nigra (2) Fraxinus pennsylvanica Dane County Ilex verticillata Fraxinus americana Prunella vulgaris Fraxinus pennsylvanica Viburnum lentago Hypericum perforatum Spiraea alba Waukesha County Grant County Dasiphora fruticosa Cephalanthus occidentalis Fraxinus nigra Cornus alternifolia Fraxinus pennsylvanica Cornus rugosa Larix laricina Rock County Eupatorium maculatum Prunella vulgaris Fraxinus pennsylvanica Fraxinus pennsylvanica Dodge County Hypericum perforatum Cornus racemosa Monarda fistulosa Columbia County Fraxinus pennsylvanica Prunella vulgaris Aronia melanocarpa Prunella vulgaris Rudbeckia hirta Carpinus caroliniana Rudbeckia laciniata Cephalanthus occidentalis Jefferson County Staphylea trifolia Cornus amomum subsp. obliqua Fraxinus pennsylvanica Viburnum lentago Fraxinus nigra Fraxinus pennsylvanica Iowa County Ilex verticillata Diervilla lonicera Spiraea alba Fraxinus nigra Viburnum lentago Fraxinus pennsylvanica Rhus typhina Map of locations where germplasm was collected Illinois Germplasm Sites Winnebago County Ptelea trifoliata Cornus alternifolia Jo Daviess County Asclepias incarnata Clematis virginiana Cornus drummondii Fraxinus pennsylvanica Rudbeckia laciniata Spiraea alba Viburnum lentago Carroll County Rudbeckia laciniata Rudbeckia triloba Hypericum perforatum -

Rain Gardens for Kalamazoo County
Patricia A.S. Crowley Office of the Kalamazoo County Drain Commissioner 201 W. Kalamazoo Avenue Rain Garden Designs for Kalamazoo, MI 49007 www.kalcounty.com/drain Kalamazoo County Installation Guidelines ∗ Visit www.raingardens.org to learn about the benefits of rain gardens. ∗ Locate the rain garden at least 10 feet from a foundaon or basement in paral or full sun, in a relavely flat area. ∗ Size the garden to be about 15-20% of the area from which it will receive runoff (e.g. roof, lawn, parking lot) for well-drained, sandy soils. Make the rain garden larger (30-45% of the drainage area) in clayey soils. ∗ Kidney, oval, or other long shapes work well, with the length about twice the width. Direct runoff into the garden’s long edge via a downspout or depression. ∗ Call MISS DIG at 811 or 1-800-482-7171 before you dig. ∗ For sandy or silty well-drained soils, dig a shallow basin about 3-6 inches deep, making the boom level and gently sloping the edges or building a berm around the lower edge. You may want to make the basin slightly deeper for clayey soils (5-7 inches). ∗ Plant nave plant plugs about 12-18 inches apart and add a 2 inch layer of ∗ To order a soil test kit self-mailer for $25 to find shredded hardwood mulch or another ground cover to suppress weeds. out your soil’s type and nutrient needs visit Using edging can help keep grass out of your garden and provide definion. hp:/bookstore.msue.msu.edu ∗ Water plants in the first two years unl established, cut back plants in the fall or spring, and divide plants and weed as needed. -

NEW PLANT SELECTIONS for 2021 ANNUALS Year of the Sunflower the Sunflower Is One of the Most Popular Genera of Flowers to Grow in Your Garden
NEW PLANT SELECTIONS FOR 2021 ANNUALS Year of the Sunflower The Sunflower is one of the most popular genera of flowers to grow in your garden. First-time to experienced gardeners gravitate to these bold, easy to grow flowers. Sunflowers originated in the Americas and domestic seeds dating back to 2100 BC have been found in Mexico. Native Americans grew sunflowers as a crop, and explorers eventually brought the flowers to Europe in the 1500s. Over the next few centuries, sunflowers became increasingly popular on the European and Asian continent, with Russian farmers growing over 2 million acres in the early 19th century (most of which was used to manufacture sunflower oil). How to Grow and Care for Sunflowers: Sunflower seeds can be direct sown after the risk of frost has passed or started indoors. Seeds should be sown ¼” to ½” deep and kept moist. Taller, larger sunflower varieties have a large taproot to keep them rooted and do not do well when they are transplanted so direct sowing of those varieties is recommended. Choose a site, or a container, in full sun, with average fertility and good drainage. https://ngb.org/year-of-the-sunflower/ Proven Winners 2021 Annual of the Year – Supertunia Mini Vista® Pink Star Meet the newest star in our annual lineup! Take a closer look at Supertunia Mini Vista® Pink Star petunia to find ideas for incorporating it into your garden and learn what it needs to thrive. There’s no denying the popularity of Supertunia Vista® Bubblegum® petunia, and we know you are going to love her “little sister” – Supertunia Mini Vista® Pink Star. -

Monarda Didyma ‘Coral Reef’ North American Native Cultivar
www.whatsnative.com Monarda didyma ‘Coral Reef’ North American Native Cultivar Monarda (mo-nard-a) Named after Dr. Nicholas Monardas (1493-1588). didyma (di-di-ma) meaning twin or in pairs; two-fold (the stamens or the leaves). Zones: 3 – 8 Flower Color: Salmon-pink Height: 3 – 4’ Spacing: 18” ‘Coral Reef’ is a bright salmon pink, taller than parent ‘Marshall’s Delight’, at 3-4’ with good mildew resistance. About the Species: Common Name: Bee Balm, Oswego Tea Family: Lamiaceae Monardas can be 15-18” tall or 5’ tall, depending on the cultivar. As part of the Mint Family, Monarda has square stems and gray-green leaves, with a slightly ‘minty’ scent. The whorled clusters of petals are in a variety of colors. Monarda didyma is known to have a calming effect on bees and yields a high amount of nectar, hence the name Bee Balm. Monarda will also attract butterflies and hummingbirds. Habitat: Native to moist open woods, bottomlands, meadows and stream banks in eastern North America. In the Garden: This plant likes to grow in average to well- drained soil in full sun. Good air circulation surrounding the plant is essential to avoiding powdery mildew. Very tolerant of heat and humidity, this plant is excellent in the mixed border in the Mid-Atlantic States. Truth or Folklore White Fawn, a young Oswego Indian maiden, was mixing a poultice of crushed leaves to soothe insect bites she obtained while walkingwith her beau, Running Wolf. Running Wolf had used Monarda to scent his pomade that was made out of (hopefully fresh) bear grease. -

Lamiales Newsletter
LAMIALES NEWSLETTER LAMIALES Issue number 4 February 1996 ISSN 1358-2305 EDITORIAL CONTENTS R.M. Harley & A. Paton Editorial 1 Herbarium, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AE, UK The Lavender Bag 1 Welcome to the fourth Lamiales Universitaria, Coyoacan 04510, Newsletter. As usual, we still Mexico D.F. Mexico. Tel: Lamiaceae research in require articles for inclusion in the +5256224448. Fax: +525616 22 17. Hungary 1 next edition. If you would like to e-mail: [email protected] receive this or future Newsletters and T.P. Ramamoorthy, 412 Heart- Alien Salvia in Ethiopia 3 and are not already on our mailing wood Dr., Austin, TX 78745, USA. list, or wish to contribute an article, They are anxious to hear from any- Pollination ecology of please do not hesitate to contact us. one willing to help organise the con- Labiatae in Mediterranean 4 The editors’ e-mail addresses are: ference or who have ideas for sym- [email protected] or posium content. Studies on the genus Thymus 6 [email protected]. As reported in the last Newsletter the This edition of the Newsletter and Relationships of Subfamily Instituto de Quimica (UNAM, Mexi- the third edition (October 1994) will Pogostemonoideae 8 co City) have agreed to sponsor the shortly be available on the world Controversies over the next Lamiales conference. Due to wide web (http://www.rbgkew.org. Satureja complex 10 the current economic conditions in uk/science/lamiales). Mexico and to allow potential partici- This also gives a summary of what Obituary - Silvia Botta pants to plan ahead, it has been the Lamiales are and some of their de Miconi 11 decided to delay the conference until uses, details of Lamiales research at November 1998. -

'Ofe Akwu' Soup Spoilage
Microbiology Research Journal International 19(3): 1-8, 2017; Article no.MRJI.32554 Previously known as British Microbiology Research Journal ISSN: 2231-0886, NLM ID: 101608140 SCIENCEDOMAIN international www.sciencedomain.org Inhibitory Potential of Ocimum gratissimum L on Bacterial Implicated in ‘Ofe Akwu’ Soup Spoilage O. C. Eruteya 1* , F. S. Ire 1 and C. C. Aneke 1 1Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria. Authors’ contributions This work was carried out in collaboration between all authors. Authors OCE and FSI designed the study. All authors participated in the laboratory analysis. Author OCE wrote the first draft of the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/MRJI/2017/32554 Editor(s): (1) Ana Cláudia Coelho, Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro, Portugal. Reviewers: (1) Vishwanadham Yerragunta, JNTU-Hydrabad, India. (2) P. Rameshthangam, Alagappa University, Karaikudi, Tamilnadu, India. (3) Julius Tibyangye, St. Augustine International University/Kampala International University, Uganda. Complete Peer review History: http://www.sciencedomain.org/review-history/18590 Received 1st March 2017 Accepted 29 th March 2017 Original Research Article th Published 11 April 2017 ABSTRACT Aims: The study evaluated the proximate composition, the bacteria present in freshly spoilt ‘ofe akwu’ soup and inhibitory potential of crude ethanol, methanol and aqueous leaf extract of Ocimum gratissimum on the resulting bacteria. Study Design: This was an analytical study in duplicate. Place and Duration of Study: Department of Microbiology, University of Port Harcourt, Niger Delta University, Amasoma, and South Africa, between July 2015 and December 2016. -

Palynological Evolutionary Trends Within the Tribe Mentheae with Special Emphasis on Subtribe Menthinae (Nepetoideae: Lamiaceae)
Plant Syst Evol (2008) 275:93–108 DOI 10.1007/s00606-008-0042-y ORIGINAL ARTICLE Palynological evolutionary trends within the tribe Mentheae with special emphasis on subtribe Menthinae (Nepetoideae: Lamiaceae) Hye-Kyoung Moon Æ Stefan Vinckier Æ Erik Smets Æ Suzy Huysmans Received: 13 December 2007 / Accepted: 28 March 2008 / Published online: 10 September 2008 Ó Springer-Verlag 2008 Abstract The pollen morphology of subtribe Menthinae Keywords Bireticulum Á Mentheae Á Menthinae Á sensu Harley et al. [In: The families and genera of vascular Nepetoideae Á Palynology Á Phylogeny Á plants VII. Flowering plantsÁdicotyledons: Lamiales (except Exine ornamentation Acanthaceae including Avicenniaceae). Springer, Berlin, pp 167–275, 2004] and two genera of uncertain subtribal affinities (Heterolamium and Melissa) are documented in Introduction order to complete our palynological overview of the tribe Mentheae. Menthinae pollen is small to medium in size The pollen morphology of Lamiaceae has proven to be (13–43 lm), oblate to prolate in shape and mostly hexacol- systematically valuable since Erdtman (1945) used the pate (sometimes pentacolpate). Perforate, microreticulate or number of nuclei and the aperture number to divide the bireticulate exine ornamentation types were observed. The family into two subfamilies (i.e. Lamioideae: bi-nucleate exine ornamentation of Menthinae is systematically highly and tricolpate pollen, Nepetoideae: tri-nucleate and hexa- informative particularly at generic level. The exine stratifi- colpate pollen). While the -
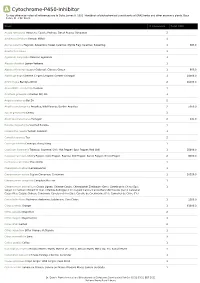
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

FEMA GRAS 29 December 2019 SUPPLEMENTARY INFORMATION 1
SUPPLEMENTARY INFORMATION 1: Identity for Natural Flavor Complexes as Evaluated by the Expert Panel The Identification Description as Reviewed by the FEMA FEMA No.1 FEMA Primary Name Expert Panel Rebaudioside M ≥80%; Rebaudioside D 5-20%; Total 4895 Rebaudioside M steviol glycosides ≥95%. Glutamic acid 35-40%; Other amino acids 1-2%; Total Corynebacterium glutamicum corn nitrogen 6-7%; Aliphatic primary alcohols, aldehydes, 4907 syrup fermentation product carboxylic acids, acetals and esters containing additional oxygenated functional groups 1-2%; Minerals 9-11% Inosine 5´-monophosphate 20-25%; Amino acids 7-8%; Corynebacterium stationis corn 4908 Minerals 23-25%; water 28-37%; Other nucleotides 1-2%; syrup fermentation product Total nitrogen 5-8% Supraglucosylated steviol glycosides 70-80%; Rebaudioside Glucosylated steviol glycosides, 4909 A 14-20%; Steviol glycosides not further glucosylated, each 70-80% individually, not to exceed 3%; Maltodextrin 3-10% Supraglucosylated steviol glycosides 30-40%; Rebaudioside Glucosylated steviol glycosides, A 5-8%; Not more than 4% stevioside; All other individual 4910 40% steviol glycosides not further glucosylated <3%; Maltodextrin 45-60% Stevioside 70-80%; Rebaudioside A 13-18%; Steviobioside 1- 3%; Rebaudioside C 2-3%; Total glycosides (including 4911 Stevia extract stevioside, 70% Rebaudioside D, Rebaudioside B, Rebaudioside F, Dulcoside A, and Rubusoside) <3% Derived from hibiscus blossom calyces (Hibiscus sabdariffa L.) , Hibiscus blossom extract is measured as water 30-60%; 4912 Hibiscus -

Seasonal Incidence of Ocimum Tingid Bug, Cochlochila Bullita Stal
Journal of Pharmacognosy and Phytochemistry 2020; 9(4): 3138-3144 E-ISSN: 2278-4136 P-ISSN: 2349-8234 www.phytojournal.com Seasonal incidence of Ocimum tingid bug, JPP 2020; 9(4): 3138-3144 Received: 07-05-2020 Cochlochila bullita Stal (Heteroptera: Tingidae) Accepted: 09-06-2020 on three different species of Ocimum viz. Ocimum Vishav Prakash Rahul basilicum L., Ocimum sanctum L. and Ocimum CSIR-Indian Institute of Integrative Medicine, Canal kilimandscharicum Guerke Road, Jammu, Jammu and Kashmir, India Vishav Prakash Rahul, Bhumika Kapoor, Sougata Sarkar and Sabha Jeet Bhumika Kapoor Project Assistant, CSIR-Indian Institute of Integrative DOI: https://doi.org/10.22271/phyto.2020.v9.i4ae.12093 Medicine, Canal Road, Jammu, Jammu and Kashmir, India Abstract The field experiment was conducted on the seasonal incidence Ocimum lace bug on three different Sougata Sarkar species of Ocimum viz. Ocimum basilicum L., Ocimum sanctum L. and Ocimum kilimandscharicum Research Associate, Scientist, Guerke at CSIR- IIIM, Chatha Farm, Jammu. The data on seasonal fluctuations of Ocimum tingid bug, CSIR-Indian Institute of Cochlochila bullita on various species of Ocimum such as Ocimum basilicum L., Ocimum sanctum L. Integrative Medicine, Canal and Ocimum kilimandscharicum Guerke were first observed during 33rd standard week of August i.e. Road, Jammu, Jammu and 0.40 Mean insect/ plant, 0.2 mean insect/plant and 0.20 mean insect/plant, respectively. The maximum Kashmir, India lace bug population was recorded on sweet basil during 39th standard week i.e. 52.60 mean insect/ plant when weekly mean maximum temperature 29.7 oC, minimum temperature 23.1 oC, morning relative Sabha Jeet Scientist, CSIR-Indian Institute humidity 93.1% and evening relative humidity 75.90 %, rainfall 93.40 mm, and wind speed 2.00 km/hr, of Integrative Medicine, Canal respectively. -

Chemical Intra-Mediterranean Variation and Insecticidal Activity of Crithmum Maritimum
Chemical Intra-Mediterranean Variation and Insecticidal Activity of Crithmum maritimum Maria Tsoukatou3, Christina Tsitsimpikoub, Constantinos Vagias 3 and Vassilios Roussis3’* a School of Pharmacy, Department of Pharmacognosy, University of Athens, Panepistimioupolis Zografou, Athens 15771, Greece. Fax: ++3017274592. E-mail: [email protected] b Doping Control Laboratory of Athens, Olympic Athletic Centre of Athens “Spiros Louis”, Kifissias 37, 15123, Maroussi, Greece * Author of correspondence and reprint requests Z. Naturforsch. 56c, 211-215 (2001); received October 27/December 7, 2000 Crithmum maritimum, Terpenes, Dillapiole, Ant Repellency The chemical composition of the volatile metabolites of Crithmum maritimum harvested from several geographic localities along the Mediterranean coasts was studied by GC and GC-MSD. The major oil constituents were found to be dillapiole, y-terpinene, sabinene, limo- nene and ß-phellandrene. The Western populations were richer in dillapiole, whereas the Southern collections were characterized by increased amounts of thymol methyl ether and y-terpinene. The Italian chemical profiles differentiated by the significant contributions of carvacrol methyl ether and isoterpinolene. The essential oils were also investigated for their insecticidal activity and their repellency against Pheidole pallidula (Nylander) ants and found to possess significant activity. Introduction Among a large set of the initially investigated Crithmum maritimum is a halophyte and chas- plants, C. maritimum, exhibited one of the highest mophyte apiaceous plant, which grows on all the antifeedant and insecticidal activities against the world’s coastlines but is particularly abundant in Pheidole pallidula ants. Other members of the the Mediterranean countries (Coiffard et al., family Apiaceae (Umbelliferae) have been re 1993). It is also referred to as rock sapphire and cently shown to exhibit antifeedant and neuro- ac was well known to sailors since ancient years for tivity against the field slug D.