A Brief Overview of Potential Treatments for Viral Diseases Using Natural Plant Compounds: the Case of SARS-Cov
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nationalmerit-2020.Pdf
Rehabilitation Council of India ‐ National Board of Examination in Rehabilitation (NBER) National Merit list of candidates in Alphabatic Order for admission to Diploma Level Course for the Academic Session 2020‐21 06‐Nov‐20 S.No Name Father Name Application No. Course Institute Institute Name Category % in Class Remark Code Code 12th 1 A REENA PATRA A BHIMASEN PATRA 200928134554 0549 AP034 Priyadarsini Service Organization, OBC 56.16 2 AABHA MAYANK PANDEY RAMESH KUMAR PANDEY 200922534999 0547 UP067 Yuva Viklang Evam Dristibadhitarth Kalyan Sewa General 75.4 Sansthan, 3 AABID KHAN HAKAM DEEN 200930321648 0547 HR015 MR DAV College of Education, OBC 74.6 4 AADIL KHAN INTZAR KHAN 200929292350 0527 UP038 CBSM, Rae Bareli Speech & Hearing Institute, General 57.8 5 AADITYA TRIPATHI SOM PRAKASH TRIPATHI 200921120721 0549 UP130 Suveera Institute for Rehabilitation and General 71 Disabilities 6 AAINA BANO SUMIN MOHAMMAD 200926010618 0550 RJ002 L.K. C. Shri Jagdamba Andh Vidyalaya Samiti OBC 93 ** 7 AAKANKSHA DEVI LAKHAN LAL 200927081668 0550 UP044 Rehabilitation Society of the Visually Impaired, OBC 75 8 AAKANKSHA MEENA RANBEER SINGH 200928250444 0547 UP119 Swaraj College of Education ST 74.6 9 AAKANKSHA SINGH NARENDRA BAHADUR SING 201020313742 0547 UP159 Prema Institute for Special Education, General 73.2 10 AAKANSHA GAUTAM TARACHAND GAUTAM 200925253674 0549 RJ058 Ganga Vision Teacher Training Institute General 93.2 ** 11 AAKANSHA SHARMA MAHENDRA KUMAR SHARM 200919333672 0549 CH002 Government Rehabilitation Institute for General 63.60% Intellectual -

The Epic Imagination in Contemporary Indian Literature
University of South Florida Scholar Commons Graduate Theses and Dissertations Graduate School May 2017 Modern Mythologies: The picE Imagination in Contemporary Indian Literature Sucheta Kanjilal University of South Florida, [email protected] Follow this and additional works at: http://scholarcommons.usf.edu/etd Part of the South and Southeast Asian Languages and Societies Commons Scholar Commons Citation Kanjilal, Sucheta, "Modern Mythologies: The pE ic Imagination in Contemporary Indian Literature" (2017). Graduate Theses and Dissertations. http://scholarcommons.usf.edu/etd/6875 This Dissertation is brought to you for free and open access by the Graduate School at Scholar Commons. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Scholar Commons. For more information, please contact [email protected]. Modern Mythologies: The Epic Imagination in Contemporary Indian Literature by Sucheta Kanjilal A dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy with a concentration in Literature Department of English College of Arts and Sciences University of South Florida Major Professor: Gurleen Grewal, Ph.D. Gil Ben-Herut, Ph.D. Hunt Hawkins, Ph.D. Quynh Nhu Le, Ph.D. Date of Approval: May 4, 2017 Keywords: South Asian Literature, Epic, Gender, Hinduism Copyright © 2017, Sucheta Kanjilal DEDICATION To my mother: for pencils, erasers, and courage. ACKNOWLEDGEMENTS When I was growing up in New Delhi, India in the late 1980s and the early 1990s, my father was writing an English language rock-opera based on the Mahabharata called Jaya, which would be staged in 1997. An upper-middle-class Bengali Brahmin with an English-language based education, my father was as influenced by the mythological tales narrated to him by his grandmother as he was by the musicals of Broadway impressario Andrew Lloyd Webber. -

TRP Channels in Visceral Pain
Send Orders of Reprints at [email protected] 23 The Open Pain Journal, 2013, 6, (Suppl 1: M4) 23-30 Open Access TRP Channels in Visceral Pain L. Ashley Blackshaw*,1,2, Stuart M. Brierley2, Andrea M. Harrington2 and Patrick A. Hughes2 1Wingate Institute for Neurogastroenterology, Centre for Digestive Diseases, Institute of Cell and Molecular Science, Barts and The London School of Medicine and Dentistry, Queen Mary University of London; 2Nerve-Gut Research Laboratory, Discipline of Medicine, The University of Adelaide, Adelaide, South Australia, Australia 5000. Abstract: Visceral pain is both different and similar to somatic pain - different in being poorly localized and usually referred elsewhere to the body wall, but similar in many of the molecular mechanisms it employs (like TRP channels) and the specialization of afferent endings to detect painful stimuli. TRPV1 is sensitive to low pH. pH is lowest in gastric juice, which may cause severe pain when exposed to the oesophageal mucosa, and probably works via TRPV1. TRPV1 is found in afferent fibres throughout the viscera, and the TRPV1 agonist capsaicin can recapitulate symptoms experienced in disease. TRPV1 is also involved in normal mechanosensory function in the gut. Roles for TRPV4 and TRPA1 have also been described in visceral afferents, and TRPV4 is highly enriched in them, where it plays a major role in both mechanonociception and chemonociception. It may provide a visceral-specific nociceptor target for drug development. TRPA1 is also involved in mechano-and chemosensory function, but not as selectively as TRPV4. TRPA1 is colocalized with TRPV1 in visceral afferents, where they influence each other’s function. -

Studies in the Floral Anatomy of the Apocynaceae
STUDIES IN THE FLORAL ANATOMY OF THE APOCYNACEAE By V. S. Rao and Arati Ganguli Rammrain Ruia College, Bombay-19 fRcceivcd for publication on November 22, 1961) I ntroduction T h e Apocynaceae is a natural taxon having a close affinity with Asclepia- daceae. This family is characterised by a deeply iive-lobed imbricate calyx, often with glands or squamellae at the base on its inner surface; a 5-lobed contorted corolla, often hairy or appendaged within; 5 epi- petalous distinct stamens, often with an apical prolongation of the con nective ; anthers either free or adherent by viscid exudates to the stigma; granular pollen; a bicarpellary, superior to half-inferior pistil with the two carpels cither free or united in the ovary portion; and a single style. In syncarpous, bilocular ovaries in this family the placentation is axile, whereas in syncarpous unilocular states it is parietal. In the apo carpous condition the placentation is marginal. A nectariferous disk is present at the base of the gynoecium in most Apocynaceae. Schumann (1895) divided Apocynaceae into the two subfamilies, Plumeroideae and Echitoideae. In Plumeroideae the stamens are either free or only slightly attached to the stigma, the anthers are generally without tails and the seeds are usually without a coma. In Echitoideae the stamens are closely attached to the stigma, the anthers have tails, and the seeds arc generally with a coma. This subfamily is believed to show affinities with Asclepiadaceae. Woodson and Moore (1938) recognized three subfamilies, namely the Plumeroideae, Echitoideae and Apocynoideae. There have been only a few investigations on the floral anatomy o f Apocynaceae., W oodson (1930) studied this group chiefly in its general morphological aspect. -

Guide for Common Viral Diseases of Animals in Louisiana
Sampling and Testing Guide for Common Viral Diseases of Animals in Louisiana Please click on the species of interest: Cattle Deer and Small Ruminants The Louisiana Animal Swine Disease Diagnostic Horses Laboratory Dogs A service unit of the LSU School of Veterinary Medicine Adapted from Murphy, F.A., et al, Veterinary Virology, 3rd ed. Cats Academic Press, 1999. Compiled by Rob Poston Multi-species: Rabiesvirus DCN LADDL Guide for Common Viral Diseases v. B2 1 Cattle Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 2 Deer and Small Ruminants Please click on the principle system involvement Generalized viral disease Respiratory viral disease Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 3 Swine Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 4 Horses Please click on the principle system involvement Generalized viral diseases Neurological viral diseases Respiratory viral diseases Enteric viral diseases Abortifacient/neonatal viral diseases Viral infections affecting the skin Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. B2 5 Dogs Please click on the principle system involvement Generalized viral diseases Respiratory viral diseases Enteric viral diseases Reproductive/neonatal viral diseases Back to the Beginning DCN LADDL Guide for Common Viral Diseases v. -

Survival of Human Norovirus Surrogates in Juices and Their Inactivation Using Novel Methods
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 5-2011 Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods Katie Marie Horm [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Recommended Citation Horm, Katie Marie, "Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods. " Master's Thesis, University of Tennessee, 2011. https://trace.tennessee.edu/utk_gradthes/882 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Katie Marie Horm entitled "Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Food Science and Technology. Doris H. D'Souza, Major Professor We have read this thesis and recommend its acceptance: Federico M. Harte, Gina M. Pighetti Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Survival of Human Norovirus Surrogates In Juices and their Inactivation Using Novel Methods A Thesis Presented for the Master of Science Degree The University of Tennessee, Knoxville Katie Marie Horm May 2011 Acknowledgments I would like to think my major professor/advisor Dr. -

Medicinal Practices of Sacred Natural Sites: a Socio-Religious Approach for Successful Implementation of Primary
Medicinal practices of sacred natural sites: a socio-religious approach for successful implementation of primary healthcare services Rajasri Ray and Avik Ray Review Correspondence Abstract Rajasri Ray*, Avik Ray Centre for studies in Ethnobiology, Biodiversity and Background: Sacred groves are model systems that Sustainability (CEiBa), Malda - 732103, West have the potential to contribute to rural healthcare Bengal, India owing to their medicinal floral diversity and strong social acceptance. *Corresponding Author: Rajasri Ray; [email protected] Methods: We examined this idea employing ethnomedicinal plants and their application Ethnobotany Research & Applications documented from sacred groves across India. A total 20:34 (2020) of 65 published documents were shortlisted for the Key words: AYUSH; Ethnomedicine; Medicinal plant; preparation of database and statistical analysis. Sacred grove; Spatial fidelity; Tropical diseases Standard ethnobotanical indices and mapping were used to capture the current trend. Background Results: A total of 1247 species from 152 families Human-nature interaction has been long entwined in has been documented for use against eighteen the history of humanity. Apart from deriving natural categories of diseases common in tropical and sub- resources, humans have a deep rooted tradition of tropical landscapes. Though the reported species venerating nature which is extensively observed are clustered around a few widely distributed across continents (Verschuuren 2010). The tradition families, 71% of them are uniquely represented from has attracted attention of researchers and policy- any single biogeographic region. The use of multiple makers for its impact on local ecological and socio- species in treating an ailment, high use value of the economic dynamics. Ethnomedicine that emanated popular plants, and cross-community similarity in from this tradition, deals health issues with nature- disease treatment reflects rich community wisdom to derived resources. -

'Ofe Akwu' Soup Spoilage
Microbiology Research Journal International 19(3): 1-8, 2017; Article no.MRJI.32554 Previously known as British Microbiology Research Journal ISSN: 2231-0886, NLM ID: 101608140 SCIENCEDOMAIN international www.sciencedomain.org Inhibitory Potential of Ocimum gratissimum L on Bacterial Implicated in ‘Ofe Akwu’ Soup Spoilage O. C. Eruteya 1* , F. S. Ire 1 and C. C. Aneke 1 1Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria. Authors’ contributions This work was carried out in collaboration between all authors. Authors OCE and FSI designed the study. All authors participated in the laboratory analysis. Author OCE wrote the first draft of the manuscript. All authors read and approved the final manuscript. Article Information DOI: 10.9734/MRJI/2017/32554 Editor(s): (1) Ana Cláudia Coelho, Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro, Portugal. Reviewers: (1) Vishwanadham Yerragunta, JNTU-Hydrabad, India. (2) P. Rameshthangam, Alagappa University, Karaikudi, Tamilnadu, India. (3) Julius Tibyangye, St. Augustine International University/Kampala International University, Uganda. Complete Peer review History: http://www.sciencedomain.org/review-history/18590 Received 1st March 2017 Accepted 29 th March 2017 Original Research Article th Published 11 April 2017 ABSTRACT Aims: The study evaluated the proximate composition, the bacteria present in freshly spoilt ‘ofe akwu’ soup and inhibitory potential of crude ethanol, methanol and aqueous leaf extract of Ocimum gratissimum on the resulting bacteria. Study Design: This was an analytical study in duplicate. Place and Duration of Study: Department of Microbiology, University of Port Harcourt, Niger Delta University, Amasoma, and South Africa, between July 2015 and December 2016. -

Genetic Content and Evolution of Adenoviruses Andrew J
Journal of General Virology (2003), 84, 2895–2908 DOI 10.1099/vir.0.19497-0 Review Genetic content and evolution of adenoviruses Andrew J. Davison,1 Ma´ria Benko´´ 2 and Bala´zs Harrach2 Correspondence 1MRC Virology Unit, Institute of Virology, Church Street, Glasgow G11 5JR, UK Andrew Davison 2Veterinary Medical Research Institute, Hungarian Academy of Sciences, H-1581 Budapest, [email protected] Hungary This review provides an update of the genetic content, phylogeny and evolution of the family Adenoviridae. An appraisal of the condition of adenovirus genomics highlights the need to ensure that public sequence information is interpreted accurately. To this end, all complete genome sequences available have been reannotated. Adenoviruses fall into four recognized genera, plus possibly a fifth, which have apparently evolved with their vertebrate hosts, but have also engaged in a number of interspecies transmission events. Genes inherited by all modern adenoviruses from their common ancestor are located centrally in the genome and are involved in replication and packaging of viral DNA and formation and structure of the virion. Additional niche-specific genes have accumulated in each lineage, mostly near the genome termini. Capture and duplication of genes in the setting of a ‘leader–exon structure’, which results from widespread use of splicing, appear to have been central to adenovirus evolution. The antiquity of the pre-vertebrate lineages that ultimately gave rise to the Adenoviridae is illustrated by morphological similarities between adenoviruses and bacteriophages, and by use of a protein-primed DNA replication strategy by adenoviruses, certain bacteria and bacteriophages, and linear plasmids of fungi and plants. -
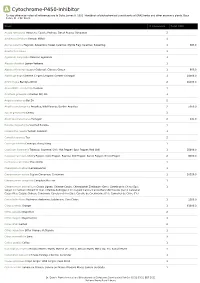
Show Activity
A Cytochrome-P450-Inhibitor *Unless otherwise noted all references are to Duke, James A. 1992. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL. CRC Press. Plant # Chemicals Total PPM Acacia farnesiana Huisache; Cassie; Popinac; Sweet Acacia; Opopanax 2 Achillea millefolium Yarrow; Milfoil 1 Acorus calamus Flagroot; Sweetroot; Sweet Calamus; Myrtle Flag; Calamus; Sweetflag 1 384.0 Agastache rugosa 1 Ageratum conyzoides Mexican ageratum 1 Aloysia citrodora Lemon Verbena 1 Alpinia officinarum Lesser Galangal; Chinese Ginger 1 800.0 Alpinia galanga Siamese Ginger; Languas; Greater Galangal 1 24000.0 Ammi majus Bishop's Weed 2 16000.0 Anacardium occidentale Cashew 1 Anethum graveolens Garden Dill; Dill 1 Angelica dahurica Bai Zhi 2 Angelica archangelica Angelica; Wild Parsnip; Garden Angelica 2 5050.0 Apium graveolens Celery 3 Artemisia dracunculus Tarragon 2 141.0 Boronia megastigma Scented Boronia 1 Calamintha nepeta Turkish Calamint 1 Camellia sinensis Tea 2 Cananga odorata Cananga; Ylang-Ylang 1 Capsicum frutescens Tabasco; Cayenne; Chili; Hot Pepper; Spur Pepper; Red Chili 1 35800.0 Capsicum annuum Cherry Pepper; Cone Pepper; Paprika; Bell Pepper; Sweet Pepper; Green Pepper 2 8000.0 Centaurea calcitrapa Star-Thistle 1 Chenopodium album Lambsquarter 1 Cinnamomum verum Ceylon Cinnamon; Cinnamon 1 20320.0 Cinnamomum camphora Camphor; Ho Leaf 1 Cinnamomum aromaticum Cassia Lignea; Chinese Cassia; Chinesischer Zimtbaum (Ger.); Canela de la China (Sp.); 1 Saigon Cinnamon; Chinazimt (Ger.); Kashia-Keihi -

Medicinal Plants Research
V O L U M E -III Glimpses of CCRAS Contributions (50 Glorious Years) MEDICINAL PLANTS RESEARCH CENTRAL COUNCIL FOR RESEARCH IN AYURVEDIC SCIENCES Ministry of AYUSH, Government of India New Delhi Illllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllll Glimpses of CCRAS contributions (50 Glorious years) VOLUME-III MEDICINAL PLANTS RESEARCH CENTRAL COUNCIL FOR RESEARCH IN AYURVEDIC SCIENCES Ministry of AYUSH, Government of India New Delhi MiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiiM Illllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllllll © Central Council for Research in Ayurvedic Sciences Ministry of AYUSH, Government of India, New Delhi - 110058 First Edition - 2018 Publisher: Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, Government of India, New Delhi, J. L. N. B. C. A. H. Anusandhan Bhavan, 61-65, Institutional Area, Opp. D-Block, Janakpuri, New Delhi - 110 058, E-mail: [email protected], Website : www.ccras.nic.in ISBN : 978-93-83864-27-0 Disclaimer: All possible efforts have been made to ensure the correctness of the contents. However Central Council for Research in Ayurvedic Sciences, Ministry of AYUSH, shall not be accountable for any inadvertent error in the content. Corrective measures shall be taken up once such errors are brought -

Methods and Formulations for Treating Chronic
(19) TZZ ¥__T (11) EP 2 346 519 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61P 1/16 (2006.01) A61K 36/537 (2006.01) 02.12.2015 Bulletin 2015/49 (86) International application number: (21) Application number: 09740785.2 PCT/US2009/059389 (22) Date of filing: 02.10.2009 (87) International publication number: WO 2010/040058 (08.04.2010 Gazette 2010/14) (54) METHODS AND FORMULATIONS FOR TREATING CHRONIC LIVER DISEASE VERFAHREN UND FORMULIERUNGEN ZUR BEHANDLUNG VON CHRONISCHER LEBERERKRANKUNG METHODES ET PREPARATIONS PERMETTANT DE TRAITER UNE MALADIE HEPATIQUE CHRONIQUE (84) Designated Contracting States: (72) Inventor: Zabrecky, George AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Ridgefield, CT 06877 (US) HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL PT RO SE SI SK SM TR (74) Representative: Bernstein, Claire Jacqueline Cabinet Orès (30) Priority: 02.10.2008 US 102110 P 36, rue de Saint Pétersbourg 75008 Paris (FR) (43) Date of publication of application: 27.07.2011 Bulletin 2011/30 (56) References cited: EP-A1- 1 637 153 WO-A1-2004/096252 (73) Proprietor: Zabrecky, George US-A1- 2003 044 512 US-A1- 2008 160 042 Ridgefield, CT 06877 (US) Note: Within nine months of the publication of the mention of the grant of the European patent in the European Patent Bulletin, any person may give notice to the European Patent Office of opposition to that patent, in accordance with the Implementing Regulations. Notice of opposition shall not be deemed to have been filed until the opposition fee has been paid.