Intracellular Trafficking and Secretion of Matrix Metalloproteinases During Macrophage Migration
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Linc-DYNC2H1-4 Promotes EMT and CSC Phenotypes by Acting As a Sponge of Mir-145 in Pancreatic Cancer Cells
Citation: Cell Death and Disease (2017) 8, e2924; doi:10.1038/cddis.2017.311 OPEN Official journal of the Cell Death Differentiation Association www.nature.com/cddis Linc-DYNC2H1-4 promotes EMT and CSC phenotypes by acting as a sponge of miR-145 in pancreatic cancer cells Yuran Gao1, Zhicheng Zhang2,3, Kai Li1,3, Liying Gong1, Qingzhu Yang1, Xuemei Huang1, Chengcheng Hong1, Mingfeng Ding*,2 and Huanjie Yang*,1 The acquisition of epithelial–mesenchymal transition (EMT) and/or existence of a sub-population of cancer stem-like cells (CSC) are associated with malignant behavior and chemoresistance. To identify which factor could promote EMT and CSC formation and uncover the mechanistic role of such factor is important for novel and targeted therapies. In the present study, we found that the long intergenic non-coding RNA linc-DYNC2H1-4 was upregulated in pancreatic cancer cell line BxPC-3-Gem with acquired gemcitabine resistance. Knockdown of linc-DYNC2H1-4 decreased the invasive behavior of BxPC-3-Gem cells while ectopic expression of linc-DYNC2H1-4 promoted the acquisition of EMT and stemness of the parental sensitive cells. Linc-DYNC2H1-4 upregulated ZEB1, the EMT key player, which led to upregulation and downregulation of its targets vimentin and E-cadherin respectively, as well as enhanced the expressions of CSC makers Lin28, Nanog, Sox2 and Oct4. Linc-DYNC2H1-4 is mainly located in the cytosol. Mechanically, it could sponge miR-145 that targets ZEB1, Lin28, Nanog, Sox2, Oct4 to restore these EMT and CSC-associated genes expressions. We proved that MMP3, the nearby gene of linc-DYNC2H1-4 in the sense strand, was also a target of miR-145. -

Discovery and Optimization of Selective Inhibitors of Meprin Α (Part II)
pharmaceuticals Article Discovery and Optimization of Selective Inhibitors of Meprin α (Part II) Chao Wang 1,2, Juan Diez 3, Hajeung Park 1, Christoph Becker-Pauly 4 , Gregg B. Fields 5 , Timothy P. Spicer 1,6, Louis D. Scampavia 1,6, Dmitriy Minond 2,7 and Thomas D. Bannister 1,2,* 1 Department of Molecular Medicine, Scripps Research, Jupiter, FL 33458, USA; [email protected] (C.W.); [email protected] (H.P.); [email protected] (T.P.S.); [email protected] (L.D.S.) 2 Department of Chemistry, Scripps Research, Jupiter, FL 33458, USA; [email protected] 3 Rumbaugh-Goodwin Institute for Cancer Research, Nova Southeastern University, 3321 College Avenue, CCR r.605, Fort Lauderdale, FL 33314, USA; [email protected] 4 The Scripps Research Molecular Screening Center, Scripps Research, Jupiter, FL 33458, USA; [email protected] 5 Unit for Degradomics of the Protease Web, Institute of Biochemistry, University of Kiel, Rudolf-Höber-Str.1, 24118 Kiel, Germany; fi[email protected] 6 Department of Chemistry & Biochemistry and I-HEALTH, Florida Atlantic University, 5353 Parkside Drive, Jupiter, FL 33458, USA 7 Dr. Kiran C. Patel College of Allopathic Medicine, Nova Southeastern University, 3301 College Avenue, Fort Lauderdale, FL 33314, USA * Correspondence: [email protected] Abstract: Meprin α is a zinc metalloproteinase (metzincin) that has been implicated in multiple diseases, including fibrosis and cancers. It has proven difficult to find small molecules that are capable Citation: Wang, C.; Diez, J.; Park, H.; of selectively inhibiting meprin α, or its close relative meprin β, over numerous other metzincins Becker-Pauly, C.; Fields, G.B.; Spicer, which, if inhibited, would elicit unwanted effects. -

Transmembrane/Cytoplasmic, Rather Than Catalytic, Domains of Mmp14
RESEARCH ARTICLE 343 Development 140, 343-352 (2013) doi:10.1242/dev.084236 © 2013. Published by The Company of Biologists Ltd Transmembrane/cytoplasmic, rather than catalytic, domains of Mmp14 signal to MAPK activation and mammary branching morphogenesis via binding to integrin 1 Hidetoshi Mori1,*, Alvin T. Lo1, Jamie L. Inman1, Jordi Alcaraz1,2, Cyrus M. Ghajar1, Joni D. Mott1, Celeste M. Nelson1,3, Connie S. Chen1, Hui Zhang1, Jamie L. Bascom1, Motoharu Seiki4 and Mina J. Bissell1,* SUMMARY Epithelial cell invasion through the extracellular matrix (ECM) is a crucial step in branching morphogenesis. The mechanisms by which the mammary epithelium integrates cues from the ECM with intracellular signaling in order to coordinate invasion through the stroma to make the mammary tree are poorly understood. Because the cell membrane-bound matrix metalloproteinase Mmp14 is known to play a key role in cancer cell invasion, we hypothesized that it could also be centrally involved in integrating signals for mammary epithelial cells (MECs) to navigate the collagen 1 (CL-1)-rich stroma of the mammary gland. Expression studies in nulliparous mice that carry a NLS-lacZ transgene downstream of the Mmp14 promoter revealed that Mmp14 is expressed in MECs at the tips of the branches. Using both mammary organoids and 3D organotypic cultures, we show that MMP activity is necessary for invasion through dense CL-1 (3 mg/ml) gels, but dispensable for MEC branching in sparse CL-1 (1 mg/ml) gels. Surprisingly, however, Mmp14 without its catalytic activity was still necessary for branching. Silencing Mmp14 prevented cell invasion through CL-1 and disrupted branching altogether; it also reduced integrin 1 (Itgb1) levels and attenuated MAPK signaling, disrupting Itgb1- dependent invasion/branching within CL-1 gels. -

Gene Symbol Category ACAN ECM ADAM10 ECM Remodeling-Related ADAM11 ECM Remodeling-Related ADAM12 ECM Remodeling-Related ADAM15 E
Supplementary Material (ESI) for Integrative Biology This journal is (c) The Royal Society of Chemistry 2010 Gene symbol Category ACAN ECM ADAM10 ECM remodeling-related ADAM11 ECM remodeling-related ADAM12 ECM remodeling-related ADAM15 ECM remodeling-related ADAM17 ECM remodeling-related ADAM18 ECM remodeling-related ADAM19 ECM remodeling-related ADAM2 ECM remodeling-related ADAM20 ECM remodeling-related ADAM21 ECM remodeling-related ADAM22 ECM remodeling-related ADAM23 ECM remodeling-related ADAM28 ECM remodeling-related ADAM29 ECM remodeling-related ADAM3 ECM remodeling-related ADAM30 ECM remodeling-related ADAM5 ECM remodeling-related ADAM7 ECM remodeling-related ADAM8 ECM remodeling-related ADAM9 ECM remodeling-related ADAMTS1 ECM remodeling-related ADAMTS10 ECM remodeling-related ADAMTS12 ECM remodeling-related ADAMTS13 ECM remodeling-related ADAMTS14 ECM remodeling-related ADAMTS15 ECM remodeling-related ADAMTS16 ECM remodeling-related ADAMTS17 ECM remodeling-related ADAMTS18 ECM remodeling-related ADAMTS19 ECM remodeling-related ADAMTS2 ECM remodeling-related ADAMTS20 ECM remodeling-related ADAMTS3 ECM remodeling-related ADAMTS4 ECM remodeling-related ADAMTS5 ECM remodeling-related ADAMTS6 ECM remodeling-related ADAMTS7 ECM remodeling-related ADAMTS8 ECM remodeling-related ADAMTS9 ECM remodeling-related ADAMTSL1 ECM remodeling-related ADAMTSL2 ECM remodeling-related ADAMTSL3 ECM remodeling-related ADAMTSL4 ECM remodeling-related ADAMTSL5 ECM remodeling-related AGRIN ECM ALCAM Cell-cell adhesion ANGPT1 Soluble factors and receptors -

Effect of Nanoparticles on the Expression and Activity of Matrix Metalloproteinases
Nanotechnol Rev 2018; 7(6): 541–553 Review Magdalena Matysiak-Kucharek*, Magdalena Czajka, Krzysztof Sawicki, Marcin Kruszewski and Lucyna Kapka-Skrzypczak Effect of nanoparticles on the expression and activity of matrix metalloproteinases https://doi.org/10.1515/ntrev-2018-0110 Received September 14, 2018; accepted October 11, 2018; previously 1 Introduction published online November 15, 2018 Matrix metallopeptidases, commonly known as matrix Abstract: Matrix metallopeptidases, commonly known metalloproteinases (MMPs), are zinc-dependent proteo- as matrix metalloproteinases (MMPs), are a group of pro- lytic enzymes whose primary function is the degradation teolytic enzymes whose main function is the remodeling and remodeling of extracellular matrix (ECM) compo- of the extracellular matrix. Changes in the activity of nents. ECM is a complex, dynamic structure that condi- these enzymes are observed in many pathological states, tions the proper tissue architecture. MMPs by digesting including cancer metastases. An increasing body of evi- ECM proteins eliminate structural barriers and allow dence indicates that nanoparticles (NPs) can lead to the cell migration. Moreover, by hydrolyzing extracellularly deregulation of MMP expression and/or activity both in released proteins, MMPs can change the activity of many vitro and in vivo. In this work, we summarized the current signal peptides, such as growth factors, cytokines, and state of knowledge on the impact of NPs on MMPs. The chemokines. MMPs are involved in many physiological literature analysis showed that the impact of NPs on MMP processes, such as embryogenesis, reproduction cycle, or expression and/or activity is inconclusive. NPs exhibit wound healing; however, their increased activity is also both stimulating and inhibitory effects, which might be associated with a number of pathological conditions, such dependent on multiple factors, such as NP size and coat- as diabetes, cardiovascular diseases and neurodegenera- ing or a cellular model used in the research. -

Self-Organization of Engineered Epithelial Tubules by Differential Cellular Motility
Self-organization of engineered epithelial tubules by differential cellular motility Hidetoshi Moria,1, Nikolce Gjorevskib,1, Jamie L. Inmana,1, Mina J. Bissella,2, and Celeste M. Nelsonb,2 aLife Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720; and bDepartments of Chemical Engineering and Molecular Biology, Princeton University, Princeton, NJ 08544 Edited by Kenneth Yamada, National Institutes of Health, Bethesda, MD, and accepted by the Editorial Board July 16, 2009 (received for review February 4, 2009) Patterning of developing tissues arises from a number of mecha- also in many epithelial tumors, including those from breast, lung, nisms, including cell shape change, cell proliferation, and cell sorting and colon (17–19), and confers cancer cells with the pernicious from differential cohesion or tension. Here, we reveal that differences ability to degrade and penetrate the basement membrane and in cell motility can also lead to cell sorting within tissues. Using mosaic metastasize to distant sites (20–23). Intriguingly, cells at the invasive engineered mammary epithelial tubules, we found that cells sorted front of metastatic cohorts express the highest levels of MMP14 (24, depending on their expression level of the membrane-anchored 25). Understanding how the expression pattern of this protease is collagenase matrix metalloproteinase (MMP)-14. These rearrange- determined will likely yield insights into possible mechanisms of ments were independent of the catalytic activity of MMP14 but cancer progression and invasion. absolutely required the hemopexin domain. We describe a signaling Here we present evidence to suggest that cellular rearrange- cascade downstream of MMP14 through Rho kinase that allows cells ments generated by differential cellular motility determine the to sort within the model tissues. -

Polymorphisms of the Matrix Metalloproteinase Genes
www.nature.com/scientificreports OPEN Polymorphisms of the matrix metalloproteinase genes are associated with essential hypertension in a Caucasian population of Central Russia Maria Moskalenko1, Irina Ponomarenko1, Evgeny Reshetnikov1*, Volodymyr Dvornyk2 & Mikhail Churnosov1 This study aimed to determine possible association of eight polymorphisms of seven MMP genes with essential hypertension (EH) in a Caucasian population of Central Russia. Eight SNPs of the MMP1, MMP2, MMP3, MMP7, MMP8, MMP9, and MMP12 genes and their gene–gene (epistatic) interactions were analyzed for association with EH in a cohort of 939 patients and 466 controls using logistic regression and assuming additive, recessive, and dominant genetic models. The functional signifcance of the polymorphisms associated with EH and 114 variants linked to them (r2 ≥ 0.8) was analyzed in silico. Allele G of rs11568818 MMP7 was associated with EH according to all three genetic models (OR = 0.58–0.70, pperm = 0.01–0.03). The above eight SNPs were associated with the disorder within 12 most signifcant epistatic models (OR = 1.49–1.93, pperm < 0.02). Loci rs1320632 MMP8 and rs11568818 MMP7 contributed to the largest number of the models (12 and 10, respectively). The EH-associated loci and 114 SNPs linked to them had non-synonymous, regulatory, and eQTL signifcance for 15 genes, which contributed to the pathways related to metalloendopeptidase activity, collagen degradation, and extracellular matrix disassembly. In summary, eight studied SNPs of MMPs genes were associated with EH in the Caucasian population of Central Russia. Cardiovascular diseases are a global problem of modern healthcare and the second most common cause of total mortality1,2. -

Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association Between BMI and Adult-Onset Non- Atopic
Supplementary Material DNA Methylation in Inflammatory Pathways Modifies the Association between BMI and Adult-Onset Non- Atopic Asthma Ayoung Jeong 1,2, Medea Imboden 1,2, Akram Ghantous 3, Alexei Novoloaca 3, Anne-Elie Carsin 4,5,6, Manolis Kogevinas 4,5,6, Christian Schindler 1,2, Gianfranco Lovison 7, Zdenko Herceg 3, Cyrille Cuenin 3, Roel Vermeulen 8, Deborah Jarvis 9, André F. S. Amaral 9, Florian Kronenberg 10, Paolo Vineis 11,12 and Nicole Probst-Hensch 1,2,* 1 Swiss Tropical and Public Health Institute, 4051 Basel, Switzerland; [email protected] (A.J.); [email protected] (M.I.); [email protected] (C.S.) 2 Department of Public Health, University of Basel, 4001 Basel, Switzerland 3 International Agency for Research on Cancer, 69372 Lyon, France; [email protected] (A.G.); [email protected] (A.N.); [email protected] (Z.H.); [email protected] (C.C.) 4 ISGlobal, Barcelona Institute for Global Health, 08003 Barcelona, Spain; [email protected] (A.-E.C.); [email protected] (M.K.) 5 Universitat Pompeu Fabra (UPF), 08002 Barcelona, Spain 6 CIBER Epidemiología y Salud Pública (CIBERESP), 08005 Barcelona, Spain 7 Department of Economics, Business and Statistics, University of Palermo, 90128 Palermo, Italy; [email protected] 8 Environmental Epidemiology Division, Utrecht University, Institute for Risk Assessment Sciences, 3584CM Utrecht, Netherlands; [email protected] 9 Population Health and Occupational Disease, National Heart and Lung Institute, Imperial College, SW3 6LR London, UK; [email protected] (D.J.); [email protected] (A.F.S.A.) 10 Division of Genetic Epidemiology, Medical University of Innsbruck, 6020 Innsbruck, Austria; [email protected] 11 MRC-PHE Centre for Environment and Health, School of Public Health, Imperial College London, W2 1PG London, UK; [email protected] 12 Italian Institute for Genomic Medicine (IIGM), 10126 Turin, Italy * Correspondence: [email protected]; Tel.: +41-61-284-8378 Int. -
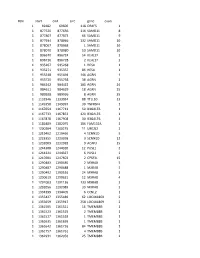
Chr Start End Size Gene Exon 1 69482 69600 118 OR4F5 1 1 877520
#chr start end size gene exon 1 69482 69600 118 OR4F5 1 1 877520 877636 116 SAMD11 8 1 877807 877873 66 SAMD11 9 1 877934 878066 132 SAMD11 10 1 878067 878068 1 SAMD11 10 1 878070 878080 10 SAMD11 10 1 896670 896724 54 KLHL17 2 1 896726 896728 2 KLHL17 2 1 935267 935268 1 HES4 1 1 935271 935357 86 HES4 1 1 955548 955694 146 AGRN 1 1 955720 955758 38 AGRN 1 1 984242 984422 180 AGRN 24 1 984611 984629 18 AGRN 25 1 989928 989936 8 AGRN 35 1 1132946 1133034 88 TTLL10 13 1 1149358 1149397 39 TNFRSF4 1 1 1167654 1167713 59 B3GALT6 1 1 1167733 1167853 120 B3GALT6 1 1 1167878 1167908 30 B3GALT6 1 1 1181889 1182075 186 FAM132A 1 1 1200204 1200215 11 UBE2J2 2 1 1219462 1219466 4 SCNN1D 5 1 1223355 1223358 3 SCNN1D 12 1 1232009 1232018 9 ACAP3 15 1 1244308 1244320 12 PUSL1 2 1 1244321 1244327 6 PUSL1 2 1 1247601 1247603 2 CPSF3L 15 1 1290483 1290485 2 MXRA8 5 1 1290487 1290488 1 MXRA8 5 1 1290492 1290516 24 MXRA8 5 1 1290619 1290631 12 MXRA8 4 1 1291003 1291136 133 MXRA8 3 1 1292056 1292089 33 MXRA8 2 1 1334399 1334405 6 CCNL2 1 1 1355427 1355489 62 LOC441869 2 1 1355659 1355917 258 LOC441869 2 1 1361505 1361521 16 TMEM88B 1 1 1361523 1361525 2 TMEM88B 1 1 1361527 1361528 1 TMEM88B 1 1 1361635 1361636 1 TMEM88B 1 1 1361642 1361726 84 TMEM88B 1 1 1361757 1361761 4 TMEM88B 1 1 1362931 1362956 25 TMEM88B 2 1 1374780 1374838 58 VWA1 3 1 1374841 1374842 1 VWA1 3 1 1374934 1375100 166 VWA1 3 1 1389738 1389747 9 ATAD3C 4 1 1389751 1389816 65 ATAD3C 4 1 1389830 1389849 19 ATAD3C 4 1 1390835 1390837 2 ATAD3C 5 1 1407260 1407331 71 ATAD3B 1 1 1407340 1407474 -

MMP19, Matrix Metalloproteinase 19 Polyclonal Antibody
MMP19, Matrix metalloproteinase 19 polyclonal antibody roteins of the matrix metalloproteinase (MMP) fam- or Research Use Only. Not for Pily are involved in the breakdown of extracellular ma- FDiagnostic or Therapeutic Use. trix in normal physiological processes, such as embryonic Purchase does not include or carry development,reproduction, and tissue remodeling, as well any right to resell or transfer this product either as a stand-alone as in disease processes, such as arthritis and metastasis. product or as a component of another Most MMP’s are secreted as inactive proproteins which are product. Any use of this product other activated when cleaved by extracellular proteinases. The than the permitted use without the function of Matrix metalloproteinase 19 (MMP19, formerly express written authorization of Allele called MMP18) has not been determined yet. The MMP19 Biotech is strictly prohibited gene codes for four different protein isoforms (rasi-1, rasi- 3, rasi-6 and rasi-9). Variant rasi-1 encodes the full length protein whereas rasi-3 uses an alternate start codon and Website: www.allelebiotech.com alternate splicing site within the coding sequence resulting Call: 1-800-991-RNAi/858-587-6645 in a much shorter protein which is translated in a different (Pacific Time: 9:00AM~5:00PM) frame. In comparison to rasi-1, rasi-6 is alternatively spliced Email: [email protected] in exon 6 producing a change in frame and a premature stop codon in exon 7. Variant rasi-9 is alternatively spliced in exon 2, producing a different in-frame start codon compared to the full length transcript and encoding a distinct 12 amino Box 1 | Basic Info acid sequence at the N-terminus of the protein, followed by sequence identical to the full length protein. -

(12) United States Patent (10) Patent No.: US 7.873,482 B2 Stefanon Et Al
US007873482B2 (12) United States Patent (10) Patent No.: US 7.873,482 B2 Stefanon et al. (45) Date of Patent: Jan. 18, 2011 (54) DIAGNOSTIC SYSTEM FOR SELECTING 6,358,546 B1 3/2002 Bebiak et al. NUTRITION AND PHARMACOLOGICAL 6,493,641 B1 12/2002 Singh et al. PRODUCTS FOR ANIMALS 6,537,213 B2 3/2003 Dodds (76) Inventors: Bruno Stefanon, via Zilli, 51/A/3, Martignacco (IT) 33035: W. Jean Dodds, 938 Stanford St., Santa Monica, (Continued) CA (US) 90403 FOREIGN PATENT DOCUMENTS (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 WO WO99-67642 A2 12/1999 U.S.C. 154(b) by 158 days. (21)21) Appl. NoNo.: 12/316,8249 (Continued) (65) Prior Publication Data Swanson, et al., “Nutritional Genomics: Implication for Companion Animals'. The American Society for Nutritional Sciences, (2003).J. US 2010/O15301.6 A1 Jun. 17, 2010 Nutr. 133:3033-3040 (18 pages). (51) Int. Cl. (Continued) G06F 9/00 (2006.01) (52) U.S. Cl. ........................................................ 702/19 Primary Examiner—Edward Raymond (58) Field of Classification Search ................... 702/19 (74) Attorney, Agent, or Firm Greenberg Traurig, LLP 702/23, 182–185 See application file for complete search history. (57) ABSTRACT (56) References Cited An analysis of the profile of a non-human animal comprises: U.S. PATENT DOCUMENTS a) providing a genotypic database to the species of the non 3,995,019 A 1 1/1976 Jerome human animal Subject or a selected group of the species; b) 5,691,157 A 1 1/1997 Gong et al. -

Computational Analyses of Small Molecules Activity from Phenotypic Screens
Computational analyses of small molecules activity from phenotypic screens Azedine Zoufir Hughes Hall This dissertation is submitted for the degree of Doctor of Philosophy July 2018 Declaration This thesis is submitted as the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. It is not substantially the same as any that I have submitted, or, is being concurrently submitted for a degree or diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the preface and specified in the text. I further state that no substantial part of my dissertation has already been submitted, or, is being concurrently submitted for any such degree, diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the Preface and specified in the text. This dissertation does not exceed the word limit of 60,000 words. Azedine Zoufir July 2018 Summary Title: Computational analyses of small molecules activity from phenotypic screens Author: Azedine Zoufir Drug discovery is no longer relying on the one gene-one disease paradigm nor on target-based screening alone to discover new drugs. Phenotypic-based screening is regaining momentum to discover new compounds since those assays provide an environment closer to the physiological state of the disease and allow to better anticipate off-target effects and other factors that can limit the efficacy of the drugs. However, uncovering the mechanism of action of the compounds active in those assays relies on in vitro techniques that are expensive and time- consuming.