Genetic Structure of Anopheles Gambiae S.S Populations Following the Use of Insecticides on Several Consecutive Years in Southern Benin Arsène Jacques Y
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Spatial Analysis of the Geographical Accessibility
IJESRT: 7(12), December, 2018 ISSN: 2277-9655 I International Journal of Engineering Sciences & Research X Technology (A Peer Reviewed Online Journal) Impact Factor: 5.164 IJESRT Chief Editor Executive Editor Dr. J.B. Helonde Mr. Somil Mayur Shah Website: www.ijesrt.com Mail: [email protected] O ISSN: 2277-9655 [Degbegnon * et al., 7(12): December, 2018] Impact Factor: 5.164 IC™ Value: 3.00 CODEN: IJESS7 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY SPATIAL ANALYSIS OF THE GEOGRAPHICAL ACCESSIBILITY TO HEALTH INFRASTRUCTURES IN THE COMMUNE OF IFANGNI IN BENIN Léopold Degbegnon*1 & Hyppolyte Lawson2 *1Engineer in Geodesy, Department of Civil Engineering of the Polytechnic School of Abomey- Calavi, University of Abomey-Calavi, Benin 2Design Engineer Topographical Surveyor, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi DOI: 10.5281/zenodo.2526183 ABSTRACT Access to health care is an essential part of an increasingly urbanized world, exposed to increasing risks as the population grows daily. The study aims to inventory infrastructures to analyse their spatial accessibility. The methodological approach used is exclusively based on the use of attribute and spatial data. The Thiessen polygon was generated to better understand the spatial coverage of health infrastructure in the Commune of Ifangni . The results obtained show that health infrastructures are unequally distributed over the territory of the municipality. In 2017, the municipality has 17 health care structures. The population travels an average of 10 km to the nearest health center. The north-east and west part of the Commune is almost devoid of health infrastructure. 81% of the communal area benefits from health care coverage at different levels. -

The Geography of Welfare in Benin, Burkina Faso, Côte D'ivoire, and Togo
Public Disclosure Authorized Public Disclosure Authorized The Geography of Welfare in Benin, Burkina Faso, Côte d’Ivoire, and Togo Public Disclosure Authorized Nga Thi Viet Nguyen and Felipe F. Dizon Public Disclosure Authorized 00000_CVR_English.indd 1 12/6/17 2:29 PM November 2017 The Geography of Welfare in Benin, Burkina Faso, Côte d’Ivoire, and Togo Nga Thi Viet Nguyen and Felipe F. Dizon 00000_Geography_Welfare-English.indd 1 11/29/17 3:34 PM Photo Credits Cover page (top): © Georges Tadonki Cover page (center): © Curt Carnemark/World Bank Cover page (bottom): © Curt Carnemark/World Bank Page 1: © Adrian Turner/Flickr Page 7: © Arne Hoel/World Bank Page 15: © Adrian Turner/Flickr Page 32: © Dominic Chavez/World Bank Page 48: © Arne Hoel/World Bank Page 56: © Ami Vitale/World Bank 00000_Geography_Welfare-English.indd 2 12/6/17 3:27 PM Acknowledgments This study was prepared by Nga Thi Viet Nguyen The team greatly benefited from the valuable and Felipe F. Dizon. Additional contributions were support and feedback of Félicien Accrombessy, made by Brian Blankespoor, Michael Norton, and Prosper R. Backiny-Yetna, Roy Katayama, Rose Irvin Rojas. Marina Tolchinsky provided valuable Mungai, and Kané Youssouf. The team also thanks research assistance. Administrative support by Erick Herman Abiassi, Kathleen Beegle, Benjamin Siele Shifferaw Ketema is gratefully acknowledged. Billard, Luc Christiaensen, Quy-Toan Do, Kristen Himelein, Johannes Hoogeveen, Aparajita Goyal, Overall guidance for this report was received from Jacques Morisset, Elisée Ouedraogo, and Ashesh Andrew L. Dabalen. Prasann for their discussion and comments. Joanne Gaskell, Ayah Mahgoub, and Aly Sanoh pro- vided detailed and careful peer review comments. -

Evaluation of the Genetic Susceptibility to the Metabolic Syndrome by the CAPN10 SNP19 Gene in the Population of South Benin
International Journal of Molecular Biology: Open Access Research Article Open Access Evaluation of the genetic susceptibility to the metabolic syndrome by the CAPN10 SNP19 gene in the population of South Benin Abstract Volume 4 Issue 6 - 2019 Metabolic syndrome is a multifactorial disorder whose etiology is resulting from the Nicodème Worou Chabi,1,2 Basile G interaction between genetic and environmental factors. Calpain 10 (CAPN10) is the first Sognigbé,1 Esther Duéguénon,1 Véronique BT gene associated with type 2 diabetes that has been identified by positional cloning with 1 1 sequencing method. This gene codes for cysteine protease; ubiquitously expressed in all Tinéponanti, Arnaud N Kohonou, Victorien 2 1 tissues, it is involved in the fundamental physiopathological aspects of insulin resistance T Dougnon, Lamine Baba Moussa and insulin secretion of type 2 diabetes. The goal of this study was to evaluate the genetic 1Department of Biochemistry and Cell Biology, University of susceptibility to the metabolic syndrome by the CAPN10 gene in the population of southern Abomey-Calavi, Benin 2 Benin. This study involved apparently healthy individuals’ aged 18 to 80 in four ethnic Laboratory of Research in Applied Biology, Polytechnic School of Abomey-Calavi, University of Abomey-Calavi, Benin groups in southern Benin. It included 74 subjects with metabolic syndrome and 323 non- metabolic syndrome patients who served as controls, with 222 women versus 175 men Correspondence: Nicodème Worou Chabi, Laboratory with an average age of 40.58 ± 14.03 years old. All subjects were genotyped for the SNP of Biochemistry and Molecular Biology, Department of 19 polymorphism of the CAPN10 gene with the PCR method in order to find associations Biochemistry and Cell Biology, Faculty of Science and between this polymorphism and the metabolic syndrome. -

Emergency Plan of Action (Epoa) Benin: Ebola Virus Disease Preparedness
Emergency Plan of Action (EPoA) Benin: Ebola Virus Disease Preparedness DREF operation Operation n° MDRBJ014; Date of Issue: 27 August 2014 Glide ° Date of disaster: 20 July 2014 Operation start date: 25 August 2014 Operation end date: 27 November ( 3 months) Host National Society(ies): Benin Red Cross Society Operation budget: CHF 50,204 Number of people affected: 14 Zones at risk Number of people assisted: One Million (indirect) 141,299 (direct) N° of National Societies involved in the operation: International Federation of Red Cross and Red Crescent Societies, Luxembourg Red Cross and Netherlands Red Cross N° of other partner organizations involved in the operation: Ministry of Health, Ministry of the Interior (through the ANPC), Plan Benin and United Nations Children’s Fund A. Situation analysis Description of the disaster In February 2014, there was an outbreak of the Ebola Virus Disease (EVD) in Guinea, which has spread to Liberia, Mali, Nigeria, Senegal and Sierra Leone causing untold hardship and hundreds of deaths in these countries. As of 27 February 2015, a total of 23,694 cases, and 9,589 deaths, which were attributed to the EVD, had been recorded across the most affected countries of Guinea, Liberia and Sierra Leone. In the Democratic Republic of Congo (DRC), an outbreak of the EVD was also reported, but is considered of a different origin than that which has affected West Africa. Benin, with a population of 10,051,000 (UNCDP 2014) shares a border with Nigeria, which has been affected by the EVD, and therefore the risks presented by the epidemic to the country are high. -

Usaid Integrated Health Services Activity (Ihsa)
Photo credit: Jocelyn Akakpo/IHSA Jocelyn credit: Photo USAID INTEGRATED HEALTH SERVICES ACTIVITY (IHSA) NEWSLETTER OCTOBER 2019 ISSUE Welcome to the first issue of the newsletter for the USAID Integrated Health Services Activity (IHSA). The information below provides key results for the first year of implementation. IHSA The purpose of the five-year IHSA, a US Agency for International The study revealed the need to strengthen the capacity of Development (USAID)-funded project implemented in Benin, is to health workers to provide quality integrated health services. For strengthen local expertise in delivering high-impact malaria, family example, only 17.2% of health centers and health zones in the planning, maternal and child health (MCH), and gender-based violence four departments were found to have a staff member trained (GBV) services, with strong citizen engagement to reduce maternal, in the integrated management of childhood illnesses. The study newborn, child, and adolescent girls’ mortality and morbidity. highlighted the low utilization of existing health services—on average, only 6.5% of women receiving at least three doses of Baseline study as the driver of future program intermittent preventive treatment of malaria in pregnancy during actions antenatal care visits. It also highlighted the need to train community health workers (CHWs) and motivate them in a sustainable way. To achieve these objectives, the program strongly emphasizes In addition, the study brought to light the need to advocate for government and citizen engagement in the health sector as well additional funding for health activities and infrastructure from as increased access to data for decision making at the local level. -

1806-IJBCS-Article-Hervé Aholoukpe Bedie
Available online at http://ajol.info/index.php/ijbcs Int. J. Biol. Chem. Sci. 7(3): 978-999, June 2013 ISSN 1991-8631 Original Paper http://indexmedicus.afro.who.int Typologie des plantations villageoises de palmier à huile ( Elaeis guineensis Jacq.) dans le département du Plateau au Bénin H. AHOLOUKPE 1,2,3*, V. P. VISSOH 2, G. AMADJI 2, P. DELEPORTE 3, B. DUBOS 4, L. NODICHAO 1, R. GLELE KAKAÏ 2, J.L. CHOTTE 5 et D. BLAVET 5 1 Centre de Recherches Agricoles Plantes Pérennes, Institut National des Recherches Agricoles du Bénin, BP 01 Pobè, Benin. 2 Faculté des Sciences Agronomiques, Université d’Abomey-Calavi, BP 526 FSA/UAC, Cotonou, Benin. 3CIRAD, UMR Eco&Sols, Place Viala, 34060 Montpellier Cedex 2, France. 4 CIRAD, UPR Systèmes de pérennes, F-34398 Montpellier, France. 5 IRD, UMR Eco&Sols, Place Viala, 34060 Montpellier Cedex 2, France. *Auteur correspondant, E-mail. [email protected]; Tel : (229) 96720404. RESUME Une typologie des palmeraies villageoises du département du Plateau au sud-est du Bénin a été établie à travers : i) une étude cartographique de la couverture pédologique et d'occupation des sols, ii) des entretiens semi-structurés en assemblée générale avec des groupes de producteurs choisis de façon aléatoire dans chaque commune, iii) des entretiens semi-structurés individuels avec quatre vingt dix producteurs dont les palmeraies sont installées sur les terres de barre et étudiées par une analyse factorielle des correspondances multiples. Les terres de barre sont les sols les plus plantés avec le palmier à huile et Adja-Ouèrè et Sakété constituent les communes de grosse production. -

Plan D'action National Budgetise Pour Le
REPUBLIQUE DU BENIN Ministère de la Santé Direction de la Santé de la Mère et l’Enfant (DSME) PLAN D’ACTION NATIONAL BUDGETISE POUR LE REPOSITIONNEMENT DE LA PLANIFICATION FAMILIALE 2014-2018 AU BENIN Décembre 2013 TABLE DES MATIERES TABLE DES MATIERES ........................................................................................ i LISTE DES ABREVIATIONS ................................................................................ iii LISTE DES TABLEAUX ........................................................................................ v LISTE DES GRAPHIQUES .................................................................................. vii PREFACE ......................................................................................................... viii INTRODUCTION .................................................................................................. 1 1- PRESENTATION DU PROCESSUS D’ELABORATION DU PLAN........................... 2 2- APERCU GENERAL SUR L’ENVIRONNEMENT DE LA PLANIFICATION FAMILIALE AU BENIN ......................................................................................... 4 2.1 Contextes démographique et sanitaire ............................................................................... 4 2.2 Options fortes faites par le Bénin dans ses documents de politique ................................... 9 2.3 Des opportunités fortes pour réussir le programme PF au Bénin..................................... 10 2.4 Avantages de la planification familiale sur les secteurs de développement -

Rapport Final De L'enquete Steps Au Benin
REPUBLIQUE DU BENIN MINISTERE DE LA SANTE Direction Nationale de la Protection Sanitaire Programme National de Lutte contre les Maladies Non Transmissibles RAPPORT FINAL DE L’ENQUETE STEPS AU BENIN Juin 2008 EQUIPE DE REDACTION Pr. HOUINATO Dismand Coordonnateur National / PNLMNT Dr SEGNON AGUEH Judith A. Médecin Epidémiologiste / PNLMNT Pr. DJROLO François Point focal diabète /PNLMNT Dr DJIGBENNOUDE Oscar Médecin Santé Publique/ PNLMNT i Sommaire RESUME ...........................................................................................................1 1 INTRODUCTION....................................................................................... 2 2 OBJECTIFS ................................................................................................ 5 3 CADRE DE L’ETUDE: (étendue géographique).........................................7 4 METHODE ............................................................................................... 16 5 RESULTATS ............................................................................................ 23 6 Références bibliographiques...................................................................... 83 7 Annexes ii Liste des tableaux Tableau I: Caractéristiques sociodémographiques des sujets enquêtés au Bénin en 2008..... 23 Tableau II: Répartition des sujets enquêtés en fonction de leur niveau d’instruction, activité professionnelle et département au Bénin en 2008. ................................................................ 24 Tableau III : Répartition des consommateurs -

Benin CO Lassa Fever
Benin CO Lassa Fever Update / 2012 / Djiwan Benin @UNICEF SITUATION IN NUMBERS Highlights UNICEF Benin is scaling up its emergency response to the ongoing Lassa Fever outbreak. As of 19 February 2016, there have been 72 cases (6 confirmed, 12 probable and 54 suspected cases), and 27 people have died (CFR 33%). As of 19 February, 479 case contacts have been identified and 478 are under 21- day surveillance. No new suspect cases were recorded and no new case contacts were reported on 19 February. One death was recorded at the CHUD-Borgou: a woman who gave birth to a healthy child the day she was admitted to the hospital on 14 February. Two suspected cases are cured and discharged from the CNHU-KM Cotonou. A car was put at disposal by Médecins du Monde to ensure the transportation of new samples from Parakou and Cotonou to Lagos reference laboratory. Daily coordination meetings at the departmental and national levels are ongoing. Continued monitoring of the contacts listed is ongoing. The 8 hospitalized cases are in good apparent clinical condition. UNICEF has provided supplies, including more than 100 hygiene kits, buckets, gloves, masks, soap, tarpaulins, water treatment units, and thermometers. UNICEF will set up an isolation and treatment center for Lassa Fever in Parakou. UNICEF leads the national Communication and Social Mobilization Commission with the production of a range of awareness raising, social mobilization and training activities, including: o community engagement and inter-personal communication (C4D) is implemented -
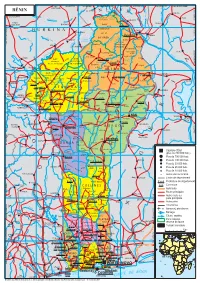
BENIN-2 Cle0aea97-1.Pdf
1° vers vers BOTOU 2° vers NIAMEY vers BIRNIN-GAOURÉ vers DOSSO v. DIOUNDIOU vers SOKOTO vers BIRNIN KEBBI KANTCHARI D 4° G vers SOKOTO vers GUSAU vers KONTAGORA I E a BÉNIN N l LA TAPOA N R l Pékinga I o G l KALGO ER M Rapides a vers BOGANDÉ o Gorges de de u JE r GA Ta Barou i poa la Mékrou KOULOU Kompa FADA- BUNZA NGOURMA DIAPAGA PARC 276 Karimama 12° 12° NATIONAL S o B U R K I N A GAYA k o TANSARGA t U DU W o O R Malanville KAMBA K Ka I bin S D É DU NIGER o ul o M k R G in u a O Garou g bo LOGOBOU Chutes p Guéné o do K IB u u de Koudou L 161 go A ZONE vers OUAGADOUGOU a ti r Kandéro CYNÉGÉTIQUE ARLI u o KOMBONGOU DE DJONA Kassa K Goungoun S o t Donou Béni a KOKO RI Founougo 309 JA a N D 324 r IG N a E E Kérémou Angaradébou W R P u Sein PAMA o PARC 423 ZONE r Cascades k Banikoara NATIONAL CYNÉGÉTIQUE é de Sosso A A M Rapides Kandi DE LA PENDJARI DE L'ATAKORA Saa R Goumon Lougou O Donwari u O 304 KOMPIENGA a Porga l é M K i r A L I B O R I 11° a a ti A j 11° g abi d Gbéssé o ZONE Y T n Firou Borodarou 124 u Batia e Boukoubrou ouli A P B KONKWESSO CYNÉGÉTIQUE ' Ségbana L Gogounou MANDOURI DE LA Kérou Bagou Dassari Tanougou Nassoukou Sokotindji PENDJARI è Gouandé Cascades Brignamaro Libant ROFIA Tiélé Ede Tanougou I NAKI-EST Kédékou Sori Matéri D 513 ri Sota bo li vers DAPAONG R Monrou Tanguiéta A T A K O A A é E Guilmaro n O Toukountouna i KARENGI TI s Basso N è s u Gbéroubou Gnémasson a Î o u è è è É S k r T SANSANN - g Kouarfa o Gawézi GANDO Kobli A a r Gamia MANGO Datori m Kouandé é Dounkassa BABANA NAMONI H u u Manta o o Guéssébani -

République Du Bénin Ministère De L'eau Et Des Mines (MEM) Société Nationale Des Eaux Du Bénin (SONEB)
République du Bénin Ministère de l'Eau et des Mines (MEM) Société Nationale des Eaux du Bénin (SONEB) Étude de collecte de données pour le développement des eaux souterraines et l’amélioration des systèmes d’approvisionnement en eau dans les départements de Couffo et Plateau en République du Bénin Rapport Final Avril 2018 Agence Japonaise de Coopération Internationale (JICA) Sanyu Consultants Inc. GE JR 18-047 Table des Matières Carte de localisation de la zone cible du Projet Table des Matières Liste des Tableaux et Figures/Abréviations/Taux de change CHAPTER 1 Présentation de l’Étude ............................................................................................... 1-1 1.1 Contexte et objectifs de l’Étude ........................................................................................... 1-1 1.2 Calendrier de l’étude .............................................................................................................. 1-1 CHAPTER 2 Présentation du secteur de l’eau Bénin .................................................................. 2-1 2.1 Plan de niveau supérieur dans le secteur de l’eau ............................................................ 2-1 2.2 Organisation du secteur de l’eau .......................................................................................... 2-1 2.3 Orientations de la coopération financière du Japon ......................................................... 2-4 2.4 Projets des autres bailleurs de fonds et organismes de coopération ............................. 2-5 CHAPTER -

N° 97-029 Du 15 Janvier 1999 Portant Organisation Des Communes En République Du Bénin
N° 97-029 du 15 Janvier 1999 Portant organisation des communes en République du Bénin L’ASSEMBLEE NATIONALE a délibéré et adopté en sa séance du 11 août 1997, en deuxième lecture de l’article 141 en sa séance des 24 juillet 1998, 24 décembre 1998 suite aux décisions : DC 98-036 des 13, 31 mars et 8 avril 1998, DC 98-080 des 07, 14 et 20 octobre 1998, pour la mise en conformité avec la Constitution. Article 1 L’Administration territoriale de la République est assurée par les autorités et services déconcentrés de l’Etat, et par les collectivités territoriales décentralisées dans le cadre défini par la présente loi. Les Circonscriptions administratives de la République du Bénin sont les départements. Il est créé en collectivité dénommée la commune. D’autres collectivités décentralisées peuvent être créées par la loi. Article 2 La présente loi fixe la dénomination et détermine le ressort territorial des structures citées ci-dessus ainsi que les règles devant régir les prérogatives des organes et personnes chargées de leur direction. Article 3 Des lois et règlements, dans leurs domaines respectifs, déterminent notamment la répartition des compétences entre les collectivités et l’Etat ainsi que la répartition des ressources publiques, les garanties statutaires accordées aux personnels des collectivités territoriales, le mode d’élection et le statut des élus, les conditions d’exercice de la tutelle par le représentant de l’Etat, le statut des grandes villes. TITRE I : DE L’ADMINISTRATION TERRITORIALE DE L’ETAT Article 4 L’Administration territoriale de l’Etat s’exerce dans le cadre du département.