Astronomy 112: the Physics of Stars Class 7 Notes: Basics of Nuclear
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Fusion with Coulomb Barrier Lowered by Scalar Field
Issue 3 (October) PROGRESS IN PHYSICS Volume 15 (2019) Nuclear Fusion with Coulomb Barrier Lowered by Scalar Field T. X. Zhang1 and M. Y. Ye2 1Department of Physics, Chemistry, and Mathematics, Alabama A & M University, Normal, Alabama 35762, USA. E-mail: [email protected] 2School of Physical Science, University of Science and Technology of China, Hefei, Anhui 230088, China. E-mail: [email protected] The multi-hundred keV electrostatic Coulomb barrier among light elemental positively charged nuclei is the critical issue for realizing the thermonuclear fusion in laborato- ries. Instead of conventionally energizing nuclei to the needed energy, we, in this paper, develop a new plasma fusion mechanism, in which the Coulomb barrier among light elemental positively charged nuclei is lowered by a scalar field. Through polarizing the free space, the scalar field that couples gravitation and electromagnetism in a five- dimensional (5D) gravity or that associates with Bose-Einstein condensates in the 4D particle physics increases the electric permittivity of the vacuum, so that reduces the Coulomb barrier and enhances the quantum tunneling probability and thus increases the plasma fusion reaction rate. With a significant reduction of the Coulomb barrier and enhancement of tunneling probability by a strong scalar field, nuclear fusion can occur in a plasma at a low and even room temperatures. This implies that the conventional fusion devices such as the National Ignition Facility and many other well-developed or under developing fusion tokamaks, when a strong scalar field is appropriately estab- lished, can achieve their goals and reach the energy breakevens only using low-techs. -

The Coulomb Barrier Not Static in QED," a Correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical Hypothesis
Frisone, F. "The Coulomb Barrier not Static in QED," A correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical hypothesis. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington, DC. “The Coulomb Barrier not Static in QED” A correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical hypothesis Fulvio Frisone Department of Physics of the University of Catania Catania, Italy, 95125, Via Santa Sofia 64 Abstract In the last two decades, irrefutable experimental evidence has shown that Low Energy Nuclear Reactions (LENR) occur in specialized heavy hydrogen systems [1-4]. Nevertheless, we are still confronted with a problem: the theoretical basis of LENR are not known and, as a matter of fact, little research has been carried out on this subject. In this work we seek to analyse the deuteron-deuteron reactions within palladium lattice by means of Preparata’s model of the palladium lattice [5,15]. We will also show the occurrence probability of fusion phenomena according to more accurate experiments [6]. We are not going to use any of the research models which have been previously followed in this field. Our aim is to demonstrate the theoretical possibility of cold fusion. Moreover, we will focus on tunneling the existent Coulomb barrier between two deuterons. Analysing the possible contributions of the lattice to the improvement of the tunneling probability, we find that there is a real mechanism through which this probability could be increased: this mechanism is the screening effect due to d-shell electrons of palladium lattice. -
Modern Physics, the Nature of the Interaction Between Particles Is Carried a Step Further
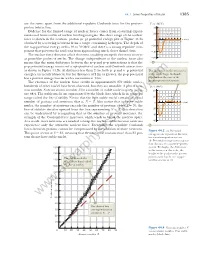
44.1 Some Properties of Nuclei 1385 are the same, apart from the additional repulsive Coulomb force for the proton– U(r ) (MeV) proton interaction. 40 Evidence for the limited range of nuclear forces comes from scattering experi- n–p system ments and from studies of nuclear binding energies. The short range of the nuclear 20 force is shown in the neutron–proton (n–p) potential energy plot of Figure 44.3a 0 r (fm) obtained by scattering neutrons from a target containing hydrogen. The depth of 1 567432 8 the n–p potential energy well is 40 to 50 MeV, and there is a strong repulsive com- Ϫ20 ponent that prevents the nucleons from approaching much closer than 0.4 fm. Ϫ40 The nuclear force does not affect electrons, enabling energetic electrons to serve as point-like probes of nuclei. The charge independence of the nuclear force also Ϫ60 means that the main difference between the n–p and p–p interactions is that the a p–p potential energy consists of a superposition of nuclear and Coulomb interactions as shown in Figure 44.3b. At distances less than 2 fm, both p–p and n–p potential The difference in the two curves energies are nearly identical, but for distances of 2 fm or greater, the p–p potential is due to the large Coulomb has a positive energy barrier with a maximum at 4 fm. repulsion in the case of the proton–proton interaction. The existence of the nuclear force results in approximately 270 stable nuclei; hundreds of other nuclei have been observed, but they are unstable. -

Binding Blocks: Building the Universe One Nucleus at the Time
Binding Blocks: building the Universe one nucleus at the time C. Aa. Diget, A. Pastore, K. Leech, T. Haylett , S. Lock, T. Sanders, M. Shelley, H. V. Willett, Department of Physics, University of York, Heslington, York, Y010 5DD, United Kingdom J. Keegans, E.A. Milne Centre for Astrophysics School of Mathematics and Physical Sciences, University of Hull, Hull, HU6 7RX, United Kingdom L. Sinclair, Castle Hill Hospital, Cottingham, HU16 5JQ, United Kingdom E. C. Simpson, Department of Nuclear Physics, Research School of Physics and Engineering, The Australian National University, Canberra ACT 2601, Australia and the Binding Blocks collaboration #JOEJOH #JOEJOH #JOEJOH #MPDLT #MPDLT #MPDLT #VJMEJOHUIF6OJWFSTF #VJMEJOHUIF6OJWFSTF #VJMEJOHUIF6OJWFSTF E-mail: [email protected] POFOVDMFVTBUBUJNF POFOVDMFVTBUBUJNF POFOVDMFVTBUBUJNF Abstract. We present a new teaching and outreach activity based around the construction of a three-dimensional chart of isotopes using LEGOr bricksz. The activity, Binding Blocks, demonstrates nuclear and astrophysical processes through a seven-meter chart of all nuclear isotopes, built from over 26,000 LEGOr bricks. It integrates A-level and GCSE curricula across areas of nuclear physics, astrophysics, arXiv:1610.02296v2 [physics.ed-ph] 18 Oct 2016 and chemistry, including: nuclear decays (through the colours in the chart); nuclear binding energy (through tower heights); production of chemical elements in the cosmos; fusion processes in stars and fusion energy on Earth; as well as links to medical physics, particularly diagnostics and radiotherapy. z LEGOr is a trademark of the LEGO Group of companies which does not sponsor, authorise or endorse the present work. Binding Blocks: building the Universe one nucleus at the time 2 1. -

IUPAC/IUPAP Provisional Report)
Pure Appl. Chem. 2018; 90(11): 1773–1832 Provisional Report Sigurd Hofmanna,*, Sergey N. Dmitrieva, Claes Fahlanderb, Jacklyn M. Gatesb, James B. Robertoa and Hideyuki Sakaib On the discovery of new elements (IUPAC/IUPAP Provisional Report) Provisional Report of the 2017 Joint Working Group of IUPAC and IUPAP https://doi.org/10.1515/pac-2018-0918 Received August 24, 2018; accepted September 24, 2018 Abstract: Almost thirty years ago the criteria that are currently used to verify claims for the discovery of a new element were set down by the comprehensive work of a Transfermium Working Group, TWG, jointly established by IUPAC and IUPAP. The recent completion of the naming of the 118 elements in the first seven periods of the Periodic Table of the Elements was considered as an opportunity for a review of these criteria in the light of the experimental and theoretical advances in the field. In late 2016 the Unions decided to estab- lish a new Joint Working Group, JWG, consisting of six members determined by the Unions. A first meeting of the JWG was in May 2017. One year later this report was finished. In a first part the works and conclusions of the TWG and the Joint Working Parties, JWP, deciding on the discovery of the now named elements are summarized. Possible experimental developments for production and identification of new elements beyond the presently known ones are estimated. Criteria and guidelines for establishing priority of discovery of these potential new elements are presented. Special emphasis is given to a description for the application of the criteria and the limits for their applicability. -

Binding Energy 1 Binding Energy
Binding energy 1 Binding energy Binding energy is the mechanical energy required to disassemble a whole into separate parts. A bound system typically has a lower potential energy than its constituent parts; this is what keeps the system together—often this means that energy is released upon the creation of a bound state. The definition above corresponds to a positive binding energy (this is often causing confusion, e.g. a prominent term in chemistry is the 'free energy of binding', which is the difference of bound and unbound state and thus negative). General idea In general, binding energy represents the mechanical work which must be done against the forces which hold an object together, disassembling the object into component parts separated by sufficient distance that further separation requires negligible additional work. At the atomic level the atomic binding energy of the atom derives from electromagnetic interaction and is the energy required to disassemble an atom into free electrons and a nucleus. Electron binding energy is a measure of the energy required to free electrons from their atomic orbits. This is more commonly known as ionization energy.[1] At the nuclear level, binding energy is also equal to the energy liberated when a nucleus is created from other nucleons or nuclei.[2][3] This nuclear binding energy (binding energy of nucleons into a nuclide) is derived from the nuclear force (residual strong interaction) and is the energy required to disassemble a nucleus into the same number of free, unbound neutrons and protons it is composed of, so that the nucleons are far/distant enough from each other so that the nuclear force can no longer cause the particles to interact.[4] In astrophysics, gravitational binding energy of a celestial body is the energy required to expand the material to infinity. -

Nuclear Mass and Stability
CHAPTER 3 Nuclear Mass and Stability Contents 3.1. Patterns of nuclear stability 41 3.2. Neutron to proton ratio 43 3.3. Mass defect 45 3.4. Binding energy 47 3.5. Nuclear radius 48 3.6. Semiempirical mass equation 50 3.7. Valley of $-stability 51 3.8. The missing elements: 43Tc and 61Pm 53 3.8.1. Promethium 53 3.8.2. Technetium 54 3.9. Other modes of instability 56 3.10. Exercises 56 3.11. Literature 57 3.1. Patterns of nuclear stability There are approximately 275 different nuclei which have shown no evidence of radioactive decay and, hence, are said to be stable with respect to radioactive decay. When these nuclei are compared for their constituent nucleons, we find that approximately 60% of them have both an even number of protons and an even number of neutrons (even-even nuclei). The remaining 40% are about equally divided between those that have an even number of protons and an odd number of neutrons (even-odd nuclei) and those with an odd number of protons and an even number of neutrons (odd-even nuclei). There are only 5 stable nuclei known which have both 2 6 10 14 an odd number of protons and odd number of neutrons (odd-odd nuclei); 1H, 3Li, 5B, 7N, and 50 23V. It is significant that the first stable odd-odd nuclei are abundant in the very light elements 2 (the low abundance of 1H has a special explanation, see Ch. 17). The last nuclide is found in low isotopic abundance (0.25%) and we cannot be certain that this nuclide is not unstable to radioactive decay with extremely long half-life. -

BETA DECAY in Β-Decay Processes, the Mass Number a Is Constant, but the Proton and Neutron Numbers Change As the Parent Decays
BETA DECAY In β-decay processes, the mass number A is constant, but the proton and neutron numbers change as the parent decays to a daughter that is better bound with lower mass. In β− decay, a neutron transforms into a proton, and an electron is emitted to conserve charge. Overall, Z Z +1.Inβ+ decay, a proton transforms into a neutron, an electron is emitted! and Z Z 1. In a third process, called electron capture or EC decay, the nucleus! − swallows an atomic electron, most likely from the atomic 1s state, and a proton converts to a neutron. The overall the e↵ect on the radioactive nucleus is the same in β+ decay. The electrons (or positrons) emitted in the first two types of β decay have continuous kinetic energies, up to an endpoint which depends on the particular species decaying, but is usually of the order of 1 MeV. The electrons (or positrons) cannot pre-exist inside the nucleus. A simple uncertainty estimate suggests that if they did, the endpoint would be much higher in energy: If it did pre-exist, then the electron would be somewhere in the nucleus beforehand, with an uncertainty in position equal to the size of the nucleus, 1/3 ∆x r0A 5 fm, in a medium mass nucleus. ⇠ ⇠ ¯h 197 MeV.fm/c The uncertainty in momentum is then ∆p = ∆x = 5 fm =40MeV/c. So the smallest momentum that the endpoint could be would correspond to 40 MeV/c. The corresponding smallest endpoint energy would be: E2 = c2p2 + m2c4 c2p2 =40MeV. -

Nuclear Binding Energy 1 Nuclear Binding Energy
Nuclear binding energy 1 Nuclear binding energy Nuclear binding energy is the energy required to split a nucleus of an atom into its component parts. The component parts are neutrons and protons, which are collectively called nucleons. The binding energy of nuclei is always a positive number, since all nuclei require net energy to separate them into individual protons and neutrons. Thus, the mass of an atom's nucleus is always less than the sum of the individual masses of the constituent protons and neutrons when separated. This notable difference is a measure of the nuclear binding energy, which is a result of forces that hold the nucleus together. Because these forces result in the removal of energy when the nucleus is formed, and this energy has mass, mass is removed from the total mass of the original particles, and the mass is missing in the resulting nucleus. This missing mass is known as the mass defect, and represents the energy released when the nucleus is formed. Nuclear binding energy can also apply to situations when the nucleus splits into fragments composed of more than one nucleon, and in this case the binding energies for the fragments (as compared to the whole) may be either positive or negative, depending on where the parent nucleus and the daughter fragments fall on the nuclear binding energy curve (see below). If new binding energy is available when light nuclei fuse, or when heavy nuclei split, either of these processes result releases the binding energy. This energy—available as nuclear energy—can be used to produce nuclear power or build nuclear weapons. -

Experimental Search for Superheavy Elements
THE HENRYK NIEWODNICZAŃSKI INSTITUTE OF NUCLEAR PHYSICS POLISH ACADEMY OF SCIENCES UL. RADZIKOWSKIEGO, 31-342 KRAKÓW, POLAND WWW.IFJ.EDU.PL/PUBL/REPORTS/2008/ KRAKÓW, DECEMBER 2008 REPORT No. 2019/PL Experimental search for superheavy elements Andrzej Wieloch* Habilitation thesis (prepared in March 2008) *M. Smoluchowski Institute of Physics, Jagiellonian University. Reymonta 4. 30-059 Kraków, e-mail: [email protected] Abstract This work reports on the experimental search for superheavy elements (SHE). Two types of approaches for SHE production are studied i.e.: "cold" fusion mech anism and massive transfer mechanism. First mechanism was studied, in normal and inverse kinematics, by using Wien filter at the GANIL facility. The production of elements with Z=106 and 108 is reported while negative result on the synthesis of SHE elements with Z=114 and 118 was received. The other approach i.e. re actions induced by heavy ion projectiles (e.g.:172Yb. 197Au) on fissile target nuclei (e.g.: 2S8U., 2S2Th) at near Coulomb barrier incident energies was studied by using superconducting solenoid installed in Texas A&M University. Preliminary results for the reaction 197 Au(7.5MeV/u) -f 232Th are presented where three cases of the possible candidates for SHE elements were found. A dedicated detection setup for such studies is discussed and the detailed data analysis is presented. Detection of al pha and spontaneous fission radioactive decays is used to unambiguously identify the atomic number of SHE. Special statistical analysis for a very low detected number of a decays is applied to check consistency of the a radioactive chains. -

1. Mass Excesses and Binding Energies the Periodic Chart of Elements Is a Useful Graphical Presentation of the Elements of Nature
1. Mass Excesses and Binding Energies The Periodic chart of Elements is a useful graphical presentation of the elements of nature. The elements are identified via the number of electrons in the neutral atom. (Z) which corresponds to the element name (e.g. Hydrogen [H] has Z = 1, Helium [He] has Z = 2 and Iron [Fe] has Z = 26). The atoms of an element consist of Z- electrons orbiting a nucleus with charge +Z. The nucleus is made of protons and neutrons. Since neutrons have no charge and protons +1 charge, there are Z-protons. A single element can have more than one isotope; when the number of protons is fixed, but the number of neutrons varies from isotope to isotope. The natural abundance of the element hydrogen is dominated by the isotope 1H, with smaller quantities of heavy hydrogen (or deuterium) 2H. The number in this notation is the associated mass number, it is the sum of the number of protons and neutrons. The element hydrogen has 1 proton, thus 1H has 0 neutrons and 2H has 1 neutron. One can see that the Periodic chart of Elements is not adequate to graphically show this richness of nuclei, thus we create what is known as a chart of nuclides, a graphical representation of nuclei. The chart is organized with the number of neutrons (N) situated on the horizontal axis and the number of protons (Z) on the vertical axis. Please visit the National Nuclear Data Center’s chart of nuclides to get familiar with the chart (http://www.nndc.bnl.gov/chart/). -

Master Thesis Armelius.Pdf (2.303Mb)
Cold nuclear fusion theories Exploration of exotic hydrogen states Joel Simion Armelius A thesis submitted in partial fulfilment of the requirements for the degree of Master of Science in the subject of Energy Technology Geophysical Institute University of Bergen Bergen, Norway April 10, 2018 Foreword As far back as 2007, I was an eager high-school student pondering and reading about different breakthrough clean energy technologies I found all around the internet. After reading wide and far, I stumbled upon cold fusion. I was at the time convinced that this energy source would very soon put an end to both war and global warming although the sea of underlying physical mechanisms proposed always gave me a headache as they never seemed to agree. When telling my friends about what I thought was an imminent energy revolution they were seemingly impressed, but as they thought we could not make a difference they didn’t pay much attention, and nothing was really done beyond that. I realised that I would need an alternative approach and in 2010 it dawned on me that others would probably use a sharper ear and be more intrigued if I had studied the subject. I was aware that studying cold fusion at a university was probably not possible based on its turbulent and stigmatized history. Of course the headaches I had previously incurred neither served as further motivation to study the disputed sea of suggested theories. I therefore concluded that I would by-pass the actual physics and instead focus on technology application, relying on physicists to develop a theory when the unexplained energy source was powering their lives.