Master Thesis Armelius.Pdf (2.303Mb)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nuclear Fusion with Coulomb Barrier Lowered by Scalar Field
Issue 3 (October) PROGRESS IN PHYSICS Volume 15 (2019) Nuclear Fusion with Coulomb Barrier Lowered by Scalar Field T. X. Zhang1 and M. Y. Ye2 1Department of Physics, Chemistry, and Mathematics, Alabama A & M University, Normal, Alabama 35762, USA. E-mail: [email protected] 2School of Physical Science, University of Science and Technology of China, Hefei, Anhui 230088, China. E-mail: [email protected] The multi-hundred keV electrostatic Coulomb barrier among light elemental positively charged nuclei is the critical issue for realizing the thermonuclear fusion in laborato- ries. Instead of conventionally energizing nuclei to the needed energy, we, in this paper, develop a new plasma fusion mechanism, in which the Coulomb barrier among light elemental positively charged nuclei is lowered by a scalar field. Through polarizing the free space, the scalar field that couples gravitation and electromagnetism in a five- dimensional (5D) gravity or that associates with Bose-Einstein condensates in the 4D particle physics increases the electric permittivity of the vacuum, so that reduces the Coulomb barrier and enhances the quantum tunneling probability and thus increases the plasma fusion reaction rate. With a significant reduction of the Coulomb barrier and enhancement of tunneling probability by a strong scalar field, nuclear fusion can occur in a plasma at a low and even room temperatures. This implies that the conventional fusion devices such as the National Ignition Facility and many other well-developed or under developing fusion tokamaks, when a strong scalar field is appropriately estab- lished, can achieve their goals and reach the energy breakevens only using low-techs. -

The Coulomb Barrier Not Static in QED," a Correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical Hypothesis
Frisone, F. "The Coulomb Barrier not Static in QED," A correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical hypothesis. in ICCF-14 International Conference on Condensed Matter Nuclear Science. 2008. Washington, DC. “The Coulomb Barrier not Static in QED” A correction to the Theory by Preparata on the Phenomenon of Cold Fusion and Theoretical hypothesis Fulvio Frisone Department of Physics of the University of Catania Catania, Italy, 95125, Via Santa Sofia 64 Abstract In the last two decades, irrefutable experimental evidence has shown that Low Energy Nuclear Reactions (LENR) occur in specialized heavy hydrogen systems [1-4]. Nevertheless, we are still confronted with a problem: the theoretical basis of LENR are not known and, as a matter of fact, little research has been carried out on this subject. In this work we seek to analyse the deuteron-deuteron reactions within palladium lattice by means of Preparata’s model of the palladium lattice [5,15]. We will also show the occurrence probability of fusion phenomena according to more accurate experiments [6]. We are not going to use any of the research models which have been previously followed in this field. Our aim is to demonstrate the theoretical possibility of cold fusion. Moreover, we will focus on tunneling the existent Coulomb barrier between two deuterons. Analysing the possible contributions of the lattice to the improvement of the tunneling probability, we find that there is a real mechanism through which this probability could be increased: this mechanism is the screening effect due to d-shell electrons of palladium lattice. -
Modern Physics, the Nature of the Interaction Between Particles Is Carried a Step Further
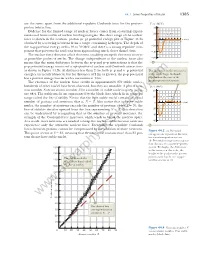
44.1 Some Properties of Nuclei 1385 are the same, apart from the additional repulsive Coulomb force for the proton– U(r ) (MeV) proton interaction. 40 Evidence for the limited range of nuclear forces comes from scattering experi- n–p system ments and from studies of nuclear binding energies. The short range of the nuclear 20 force is shown in the neutron–proton (n–p) potential energy plot of Figure 44.3a 0 r (fm) obtained by scattering neutrons from a target containing hydrogen. The depth of 1 567432 8 the n–p potential energy well is 40 to 50 MeV, and there is a strong repulsive com- Ϫ20 ponent that prevents the nucleons from approaching much closer than 0.4 fm. Ϫ40 The nuclear force does not affect electrons, enabling energetic electrons to serve as point-like probes of nuclei. The charge independence of the nuclear force also Ϫ60 means that the main difference between the n–p and p–p interactions is that the a p–p potential energy consists of a superposition of nuclear and Coulomb interactions as shown in Figure 44.3b. At distances less than 2 fm, both p–p and n–p potential The difference in the two curves energies are nearly identical, but for distances of 2 fm or greater, the p–p potential is due to the large Coulomb has a positive energy barrier with a maximum at 4 fm. repulsion in the case of the proton–proton interaction. The existence of the nuclear force results in approximately 270 stable nuclei; hundreds of other nuclei have been observed, but they are unstable. -

Astronomy 112: the Physics of Stars Class 7 Notes: Basics of Nuclear
Astronomy 112: The Physics of Stars Class 7 Notes: Basics of Nuclear Fusion In this class we continue the process of filling in the missing microphysical details that we need to make a stellar model. To recap, in the last two classes we computed the pressure of stellar material and the rate of energy transport through the star. These were two of the missing pieces we needed. The third, which we’ll sketch out over the next two lectures, is the rate for nuclear reactions, and the energy that they generate. I. Energetics A. Energy Release All nuclear reactions fundamentally work by converting mass into energy. (In some ways the same could be said of chemical reactions, but for those the masses involved are so tiny as to not be worth worrying about.) The masses of the reactants involved therefore determine the energy released by the reaction. Consider a reaction between two species that produced some other species I(Ai, Zi) + J (Aj, Zj) → K(Ak, Zk) + L(Al, Zl), where as usual Z is the atomic number and A is the atomic mass number. At this point we must distinguish between atomic mass number and actual mass, so let M be the mass of a given species. The atomic mass number times mH and the true mass are nearly identical, M ≈ AmH, but not quite, and that small difference is the source of energy for the reaction. For the reaction we have written down, the energy released is 2 Qijk = (Mi + Mj − Mk − Ml)c , i.e. the initial mass minus the final mass, multiplied by c2. -

Massive Stars As Thermonuclear Reactors and Their Explosions
Massive stars as thermonuclear reactors and their explosions following core collapse Alak Ray Tata Institute of Fundamental Research, Mumbai 400 005, India [email protected] Summary. Nuclear reactions transform atomic nuclei inside stars. This is the pro- cess of stellar nucleosynthesis. The basic concepts of determining nuclear reaction rates inside stars are reviewed. How stars manage to burn their fuel so slowly most of the time are also considered. Stellar thermonuclear reactions involving protons in hydrostatic burning are discussed first. Then I discuss triple alpha reactions in the helium burning stage. Carbon and oxygen survive in red giant stars because of the nuclear structure of oxygen and neon. Further nuclear burning of carbon, neon, oxygen and silicon in quiescent conditions are discussed next. In the subse- quent core-collapse phase, neutronization due to electron capture from the top of the Fermi sea in a degenerate core takes place. The expected signal of neutrinos from a nearby supernova is calculated. The supernova often explodes inside a dense circumstellar medium, which is established due to the progenitor star losing its out- ermost envelope in a stellar wind or mass transfer in a binary system. The nature of the circumstellar medium and the ejecta of the supernova and their dynamics are revealed by observations in the optical, IR, radio, and X-ray bands, and I discuss some of these observations and their interpretations. Keywords: nuclear reactions, nucleosynthesis, abundances; stars: interiors; supernovae: general; neutrinos; circumstellar matter; X-rays: stars 1 Introduction The sun is not commonly considered a star and few would think of stars as nuclear reactors. -

Enhanced D-D Fusion Rates When the Coulomb Barrier Is Lowered by Electrons Alfred Y
Enhanced D-D Fusion Rates when the Coulomb Barrier Is Lowered by Electrons Alfred Y. Wonga*, Alexander Gunna, Allan X. Chena,b, Chun-Ching Shihc, Mason J. Guffeya May 20, 2021 a (Alpha Ring International Limited: Monterey, CA, USA) b (Adelphi Technologies, Inc: Redwood City, CA, USA) c (Chun-Ching Shih: CA, USA) * Corresponding Author: [email protected] Abstract A profusion of unbound, low-energy electrons creates a local electric field that reduces Coulomb potential and increases quantum tunneling probability for pairs of nuclei. Neutral beam-target experiments on deuterium-deuterium fusion reactions, observed with neutron detectors, show percentage increases in fusion products are consistent with electron-screening predictions from Schrödinger wave mechanics. Experiments performed confirm that observed fusion rate enhancement with a negatively biased target is primarily due to changes to the fusion cross section, rather than simply acceleration due to electrostatic forces. PACS indices: 52.30.-q; 25.30.-c; 25.70.Jj Key words: electron shielding; fusion cross section; shielding by free electrons; D-D fusion; neutral beam 1 Confidential – Proprietary Information of Alpha Ring International Limited 1. Introduction The effect of electrons on fusion reaction rates has been investigated for many years to understand its influence on stellar nucleosynthesis. The screening effects due to bound electrons have been studied for various nuclear reactions, including D-D, D-3He, 3He-3He, p-11B, and p-7Li [1–6]. For the reaction p-11B, the screened cross section has been measured for energies between 17 and 134 keV [1]. In this range, the screening potential is only 0.25 to 2 percent of the total interaction energy, resulting in enhancement of fusion cross sections of less than 10 percent [5]. -

Electrostatic Application Principles
International PhD Seminar on Computational electromagnetics and optimization in electrical engineering – CEMOEE 2010 10-13 September, Sofia, Bulgaria ELECTROSTATIC APPLICATION PRINCIPLES invited paper Mićo GAĆANOVIĆ "Jede Ursache hat ihre Wirkung; jede Wirkung ihre Ursache; an explanation of ultimate substance, change, and the exis- alles geschieht gesetzmäßig, Zufall ist nur ein Name für ein tence of the world -- without reference to mythology. Those unbekanntes Gesetz. Es gibt viele Ebenen der Ursächlichkeit, aber philosophers were also influential, and eventually Thales' nichts entgeht dem Gesetz." rejection of mythological explanations became an essential “Kybalion” idea for the scientific revolution. He was also the first to define general principles and set forth hypotheses, and as a result has been dubbed the "Father of Science". HISTORY In mathematics, Thales used geometry to solve prob- lems such as calculating the height of pyramids and the Thales of Miletus Thales of Miletus (Θαλῆς ὁ distance of ships from the shore. He is credited with the Μιλήσιος) Born ca. 624–625 BC Died ca. 547–546 BC , first use of deductive reasoning applied to geometry, by [16]. deriving four corollaries to Thales' Theorem. As a result, he As reported by the Ancient Greek philosopher Thales of has been hailed as the first true mathematician and is the Miletus around 600 B.C.E., charge (or electricity) could be first known individual to whom a mathematical discovery accumulated by rubbing fur on various substances, such as has been attributed. amber. The Greeks noted that the charged amber buttons In 1600, the English scientist William Gilbert returned could attract light objects such as hair. -
![Arxiv:1604.03197V2 [Nucl-Th] 4 May 2016](https://docslib.b-cdn.net/cover/4594/arxiv-1604-03197v2-nucl-th-4-may-2016-6884594.webp)
Arxiv:1604.03197V2 [Nucl-Th] 4 May 2016
Frontiers in Nuclear Astrophysics C. A. Bertulani a;b and T. Kajino c;d aDepartment of Physics and Astronomy, Texas A & M University-Commerce, Commerce, Texas 75429, USA bDepartment of Physics and Astronomy, Texas A&M University, College Station, Texas 77843, USA cNational Astronomical Observatory of Japan, 2-21-1 Osawa, Mitaka, Tokyo 181-8588, Japan dDepartment of Astronomy, Graduate School of Science, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo, 113-0033, Japan Abstract The synthesis of nuclei in diverse cosmic scenarios is reviewed, with a summary of the basic concepts involved before a discussion of the current status in each case is made. We review the physics of the early universe, the proton to neutron ratio influence in the observed helium abundance, reaction networks, the formation of elements up to beryllium, the inhomogeneous Big Bang model, and the Big Bang nucleosynthesis constraints on cosmological models. Attention is paid to element production in stars, together with the details of the pp chain, the pp reaction, 3He formation and destruction, electron capture on 7Be, the importance of 8B formation and its relation to solar neutrinos, and neutrino oscillations. Nucleosynthesis in massive stars is also reviewed, with focus on the CNO cycle and its hot companion cycle, the rp-process, triple-α capture, and red giants and AGB stars. The stellar burning of carbon, neon, oxygen, and silicon is presented in a separate section, as well as the slow and rapid nucleon capture processes and the importance of medium modifications due to electrons also for pycnonuclear reactions. The nucleosynthesis in cataclysmic events such as in novae, X-ray bursters and in core-collapse supernovae, the role of arXiv:1604.03197v2 [nucl-th] 4 May 2016 neutrinos, and the supernova radioactivity and light-curve is further discussed, as well as the structure of neutron stars and its equation of state. -

15 Electric Charge and Electric Field
Electric Charge and Electric Field MODULE - 5 Electricity and Magnetism 15 Notes ELECTRIC CHARGE AND ELECTRIC FIELD So far you have learnt about mechanical, thermal and optical systems and various phenomena exhibited by them. The importance of electricity in our daily life is too evident. The physical comforts we enjoy and the various devices used in daily life depend on the availability of electrical energy. An electrical power failure demonstrates directly our dependence on electric and magnetic phenomena; the lights go off, the fans, coolers and air-conditioners in summer and heaters and gysers in winter stop working. Similarly, radio, TV, computers, microwaves can not be operated. Water pumps stop running and fields cannot be irrigated. Even train services are affected by power failure. Machines in industrial units can not be operated. In short, life almost comes to a stand still, sometimes even evoking public anger. It is, therefore, extremely important to study electric and magnetic phenomena. In this lesson, you will learn about two kinds of electric charges, their behaviour in different circumstances, the forces that act between them, the behaviour of the surrounding space etc. Broadly speaking, we wish to study that branch of physics which deals with electrical charges at rest. This branch is called electrostatics. OBJECTIVES After studying this lesson, you should be able to : z state the basic properties of electric charges; z explain the concepts of quantisation and conservation of charge; z explain Coulomb’s law of force between electric charges; z define electric field due to a charge at rest and draw electric lines of force; z define electric dipole, dipole moment and the electric field due to a dipole; PHYSICS 1 MODULE - 5 Electric Charge and Electric Field Electricity and Magnetism z state Gauss’ theorem and derive expressions for the electric field due to a point charge, a long charged wire, a uniformly charged spherical shell and a plane sheet of charge; and z describe how a van de Graaff generator functions. -

Overcoming the Coulomb Barrier in Cold Fusion
J. Condensed Matter Nucl. Sci. 2 (2009) 51–59 Research Article Overcoming the Coulomb Barrier in Cold Fusion Talbot A. Chubb ∗ 5023 N. 38th St. Arlington, VA 22207, USA Scott R. Chubb † 903 Frederick St., Apt. 6, Arlington VA, 22204-6611, USA Abstract Schwinger pointed out that under some circumstances the Coulomb barrier between paired charged particles is replaced by a correlation factor in a two-body wave function. This paper shows how having two deuterons bound within a common volume having a multiplicity of potential wells can lead to an energy-minimized Schwinger form of wave equation with wave function overlap. Relevance to a situation in which a small number of deuterium atoms is forced into a fully loaded palladium deuteride (PdD) host is discussed. © 2009 ISCMNS. All rights reserved. Keywords: Bloch state ions, Catalytic nuclear fusion, Coherent partitioning, Cold fusion, Ion band state theory 1. Introduction In conventional nuclear fusion [1], the presence of a Coulomb barrier between interacting nucleons has always prevented nuclear reactions, except at high collisional energy or in the presence of negative muons. Reaction rates between deuterons are calculated by use of the Gamow factor. When the Gamow factor is applied to deuterons at condensed matter temperatures, it forces reaction rates to be much too low to be observable. Nonetheless, Julian Schwinger believed that the Gamow factor models were wrongly applied to cold fusion experiments. He stated, “In the very low energy cold fusion, one deals essentially with a single state, described by a single-wave function, all parts of which are coherent. A separation into two independent, incoherent factors is not possible, and all considerations based on such a factorization are not relevant [2]”. -

Approach to Nuclear Fusion Utilizing Dynamics of High-Density Electrons and Neutrals Alfred Y
Approach to Nuclear Fusion Utilizing Dynamics of High-Density Electrons and Neutrals Alfred Y. Wong* and Chun-Ching Shih Alpha Ring US Inc., 5 Harris Court, Building B, Monterey, California 93940 *Correspondence author: [email protected] 8/14/2019 Abstract An approach to achieve nuclear fusion utilizing the formation of high densities of electrons and neutrals is described. The profusion of low energy electrons provides high dynamic electric fields that help reduce the Coulomb barrier in nuclear fusion; high-density neutrals provide the stability and reaction rates to achieve break-even fusion where charged particles are the main products. Interactions of energetic charged particles with high-density background produce positive feedbacks with enhanced cross sections. Experiments in a rotating geometry illustrate the advantages of this approach, which discriminates against neutronic fusion. PACS indices: 52.30.-q; 25.30.-c; 25.70.Jj Key words: ion-neutral coupling; proton-boron fusion cross sections; static and dynamic electron screening; power gain and fusion products I. Introduction Conventional nuclear fusion efforts are generally concentrated at overcoming the Coulomb barrier through the confinement of energetic ions of tens to hundreds of keV. This paper describes a different approach through lowering the Coulomb barrier by a profusion of electrons to allow fusion to occur at low energies of tens of eV to several keV and high densities of reactants. New methods of compressing neutrals to high densities in presence of shielding electric fields encourage significant fusion processes to take place through quantum tunneling in a stable environment. 1 Positive feedbacks, which greatly enhance the fusion process, are made possible in the presence of such high-density operations. -

Abstract Constraining the P Process: Cross Section Measurement Of
CONSTRAINING THE P PROCESS: CROSS SECTION MEASUREMENT OF 84Kr(p; γ)85Rb By Alicia R Palmisano A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Physics - Doctor of Philosophy 2021 ABSTRACT CONSTRAINING THE P PROCESS: CROSS SECTION MEASUREMENT OF 84Kr(p; γ)85Rb By Alicia R Palmisano One of the biggest questions in nuclear astrophysics is understanding where the elements come from and how they are made. Everything around us, from our pets to our food to our computers, was made from material originally created in a star. There are so many aspects to consider when trying to answer these questions: "What astrophysical events make what elements?", "How many elements are made?", "How much of each element is made?" and more. The first step in answering these questions is running simulations of these astrophysical events. However, in order to get the correct final abundances of the elements from these events huge amounts of nuclear data is required that has never been measured. This work focuses on the p process and the elements created in this process known as the p nuclei. The p process is responsible for making the stable isotopes on the proton rich side of stability and is thought to occur in core collapse or Type Ia supernova. Currently scientists rely heavily on theory to define parameters in astrophysical and nuclear reaction codes. In addition, experiments are being performed to slowly measure more nuclear data to add constraints to theory for the p process reaction networks. The focus of this thesis was an experiment that was performed with the Summing Sodium Iodide (NaI) (SuN) detector at the National Superconducting Cyclotron Facility (NSCL) at Michigan State University (MSU) using the ReAccelerator (ReA) facility.