A COOLING CURVE Pre-Lab Discussion in This Lab, You Will Be Measuring the Temperature of a Pure Substance As It Cools and Solidifies
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
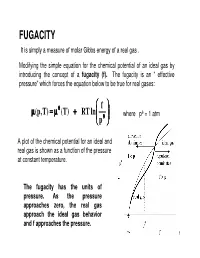
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene-Ethylbenzene
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1940 A Study of the Equilibrium Diagrams of the Systems, Benzene- Toluene and Benzene-Ethylbenzene John B. Mullen Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Chemistry Commons Recommended Citation Mullen, John B., "A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene- Ethylbenzene" (1940). Master's Theses. 299. https://ecommons.luc.edu/luc_theses/299 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1940 John B. Mullen A S'l'IJDY OF THE EQUTI.IBRIUM DIAGRAMS OF THE SYSTEMS, BENZENE-TOLUENE AND BENZENE-~ENZENE By John B. Mullen Presented in partial :f'u.J.tilment at the requirements :f'or the degree o:f' Master o:f' Science, Loyola University, 1940. T.ABLE OF CONTENTS Page Acknowledgment 1i Vita iii Introduction 1 I. Review of Literature 2 II. Apparatus and its Calibration 3 III. Procedure am Technique 6 IV. Observations on the System Benzene-Toluene 9 V. Observations on the System Benzene-Ethylbenzene 19 VI. Equilibrium Diagrams of the Systems 30 Recommendations for Future Work 34 Bibliography 35 ACKNOWLEOOMENT The au1hor wishes to acknowledge with thanks the invaluable assistance, suggestions, and cooperation offered by Dr. -

Phase Diagrams
Module-07 Phase Diagrams Contents 1) Equilibrium phase diagrams, Particle strengthening by precipitation and precipitation reactions 2) Kinetics of nucleation and growth 3) The iron-carbon system, phase transformations 4) Transformation rate effects and TTT diagrams, Microstructure and property changes in iron- carbon system Mixtures – Solutions – Phases Almost all materials have more than one phase in them. Thus engineering materials attain their special properties. Macroscopic basic unit of a material is called component. It refers to a independent chemical species. The components of a system may be elements, ions or compounds. A phase can be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics i.e. it is a physically distinct from other phases, chemically homogeneous and mechanically separable portion of a system. A component can exist in many phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond. Mixtures – Solutions – Phases (contd…) When two phases are present in a system, it is not necessary that there be a difference in both physical and chemical properties; a disparity in one or the other set of properties is sufficient. A solution (liquid or solid) is phase with more than one component; a mixture is a material with more than one phase. Solute (minor component of two in a solution) does not change the structural pattern of the solvent, and the composition of any solution can be varied. In mixtures, there are different phases, each with its own atomic arrangement. It is possible to have a mixture of two different solutions! Gibbs phase rule In a system under a set of conditions, number of phases (P) exist can be related to the number of components (C) and degrees of freedom (F) by Gibbs phase rule. -

Neutron Star Cooling and Discuss the Main Issue: What We Can Learn About the Internal Structure of Neutron Stars by Confronting Theory and Observation
To appear in Ann. Rev. Astron. Astrophys. 2004 Neutron Star Cooling D. G. Yakovlev 1 and C. J. Pethick 2 1Ioffe Physical Technical Institute, Politekhnicheskaya 26, 194021 St.-Petersburg, Russia, e-mail: [email protected]ffe.ru 2NORDITA, The Nordic Institute for Theoretical Physics, Blegdamsvej 17, DK– 2100 Copenhagen Ø, Denmark, e-mail: [email protected] Abstract Observation of cooling neutron stars can potentially provide information about the states of matter at supernuclear densities. We review physical properties important for cooling such as neutrino emission processes and superfluidity in the stellar interior, surface envelopes of light elements due to accretion of matter and strong surface magnetic fields. The neutrino pro- cesses include the modified Urca process, and the direct Urca process for nucleons and exotic states of matter such as a pion condensate, kaon con- densate, or quark matter. The dependence of theoretical cooling curves on physical input and observations of thermal radiation from isolated neutron stars are described. The comparison of observation and theory leads to a unified interpretation in terms of three characteristic types of neutron stars: high-mass stars which cool primarily by some version of the direct Urca process; low-mass stars, which cool via slower processes; and medium-mass stars, which have an intermediate behavior. The related problem of thermal states of transiently accreting neutron stars with deep crustal burning of accreted matter is discussed in connection with observations of soft X-ray transients. arXiv:astro-ph/0402143v1 6 Feb 2004 1 INTRODUCTION Neutron stars are the most compact stars in the Universe. They have masses M ∼ 1.4 M⊙ and radii R ∼ 10 km, and they contain matter at supernuclear densities in their cores. -

Cooling Electrons in Semiconductor Devices: a Model of Evaporative Emission
PHYSICAL REVIEW B 75, 035316 ͑2007͒ Cooling electrons in semiconductor devices: A model of evaporative emission Thushari Jayasekera, Kieran Mullen, and Michael A. Morrison* Homer L. Dodge Department of Physics and Astronomy, The University of Oklahoma, 440 West Brooks Street, Norman, Oklahoma 73019-0225, USA ͑Received 4 April 2006; revised manuscript received 2 November 2006; published 11 January 2007͒ We discuss the theory of cooling electrons in solid-state devices via “evaporative emission.” Our model is based on filtering electron subbands in a quantum-wire device. When incident electrons in a higher-energy subband scatter out of the initial electron distribution, the system equilibrates to a different chemical potential and temperature than those of the incident electron distribution. We show that this re-equilibration can cause considerable cooling of the system. We discuss how the device geometry affects the final electron temperatures, and consider factors relevant to possible experiments. We demonstrate that one can therefore induce substantial electron cooling due to quantum effects in a room-temperature device. The resulting cooled electron population could be used for photodetection of optical frequencies corresponding to thermal energies near room temperature. DOI: 10.1103/PhysRevB.75.035316 PACS number͑s͒: 73.50.Lw, 73.23.Ϫb I. INTRODUCTION achieve electron cooling. We improve upon this result by switching to a “plus-junction” design, which improves the As electronic devices become smaller, they leave the re- cooling characteristics of the device. In Sec. IV we discuss gime of classical physics and enter the realm of quantum applications and realistic parameters for a device to cool physics. -

Exam3 Practice
Materials Science and Engineering Department MSE 360, Test #3 ID number _______________________________ Name: _____________________________________ No notes, books, or information stored in calculator memories may be used. Cheating will be punished severely. All of your work must be written on these pages and turned in. Constants, equations, and other data are given on the last page of the exam. _______________________________________________________________________________ Problem 1-18: 2 points each (Key:1C2B3B4B5A6D 7B8D9B10B11A12A 13A14A15A16B17A18A) 1. A regular solution will likely form an ordered atomic structure if: d.) Ω = 0 b.) Ω > 0 c.) Ω < 0 2. With increasing temperature and constant pressure, the Gibbs free energy of a single phase A) increase, B) decrease 3. During annealing, the grain #2 represented in the right figure will A) be stable B) grow C) shrink 4. The mechanism of diffusion for a dilute solute atom of small atomic radius compared to the host material would be best described by: a.) Substitutional diffusion b.) Interstitial diffusion c.) Vacancy diffusion d.) all of the above 5. When Lf/Tm > 4R, where the Lf is the latent heat of fusion, Tm is the melting temperature, the liquid/solid interface is A) Smooth, B) Rough 6. The inhomogeneous nucleation is easier than the homogeneous nucleation because the inhomogeneous nucleation A. Has a critical nucleus with smaller diameter B. Has a critical nucleus with smaller volume C. Has a critical nucleus with smaller critical Gibbs free energy D. Both B and C 7. For heterogeneous nucleation in the grain interior, grain boundaries, grain edges and grain corners, their critical energy barriers can be described as A. -

COOLING CURVE ANALYSIS in BINARY Al-Cu ALLOYS: PART I- EFFECT of COOLING RATE and COPPER CONTENT on the EUTECTIC FORMATION
Association of Metallurgical Engineers of Serbia Scientific paper AMES UDC: 669.715.018 COOLING CURVE ANALYSIS IN BINARY Al-Cu ALLOYS: PART I- EFFECT OF COOLING RATE AND COPPER CONTENT ON THE EUTECTIC FORMATION M. Dehnavi1, F. Kuhestani2, M. Haddad-Sabzevar1 1Department of Materials Engineering and Metallurgy, Ferdowsi University of Mashhad, Mashhad, Iran 2Faculty of Materials Engineering, Semnan University, Semnan, Iran. Received 28.02.2015 Accepted 25.08.2015 Abstract There are many techniques available for investigating the solidification of metals and alloys. In recent years computer-aided cooling curve analysis (CA-CCA) has been used to determine thermo-physical properties of alloys, latent heat and solid fraction. In this study, the effect of cooling rate and copper addition was taken into consideration in non- equilibrium eutectic transformation of binary Al- Cu melt via cooling curve analysis. For this purpose, melts with different copper weight percent of 2.2, 3.7 and 4.8 were prepared and cooled in controlled rates of 0.04 and 0.42 °C/s. Results show that, latent heat of alloy highly depends upon the post- solidification cooling rate and composition. As copper content of alloy and cooling rate increase, achieved non- equilibrium eutectic phase increases that leads to release of high amount of latent heat and appearing of second deviation in cooling curve. This deviation can be seen in first time derivative curve in the form of a definite peak. Key words: cooling curve, latent heat, first derivative curve, non- equilibrium eutectic Introduction Depending on the casting conditions and alloy composition, microstructure, properties and characteristics of the aluminum alloys will be different [1]. -

A DSC-Study on the Demixing of Binary Polymer Solutions
A DSC-study on the demixing of binary polymer solutions Peter van der Heijden A DSC-STUDY ON THE DEMIXING OF BINARY POLYMER SOLUTIONS PROEFSCHRIFT ter verkrijging van de graad van doctor aan de Universiteit Twente, op gezag van de rector magnificus, prof. dr. F.A. van Vught, volgens het besluit van het College voor Promoties in het openbaar te verdedigen op vrijdag 12 oktober te 15.00 uur door Petrus Cornelis van der Heijden geboren op 16 augustus 1973 te Hoogland Dit proefschrift is goedgekeurd door de promotoren: prof.dr.-ing M. Wessling en prof.dr.ing M.H.V.Mulder This research was financially supported by the Nederlandse organisatie voor Wetenschappelijk Onderzoek (NWO) ISBN: 90 365 1646 3 2001 by P.C. van der Heijden All rights reserved. Printed by PrintPartners Ipskamp, Enschede Cover design: Carolien van der Heijden Contents Chapter 1 1 Introduction Chapter 2 9 Quenching of concentrated polymer-diluent systems Appendix 2: Heat transfer in a DSC-pan 34 Chapter 3 39 Phase behavior of polymer-diluent systems characterized by temperature modulated differential scanning calorimetry Chapter 4 55 Quantification and interpretation of temperature modulated differential scanning calorimetry data on liquid-liquid demixing and vitrification Appendix 4A: Verification of the assumptions proposed in Chapter 4 72 * Appendix 4B: Physical significance of the interpolated heat capacity cp i 82 Chapter 5 89 Diluent crystallization and melting in a liquid-liquid demixed and vitrified polymer solutions Appendix 5: Comments on the concept of thermoporometry 118 Chapter 6 129 Determination of a binary phase diagram with one single temperature modulated differential scanning calorimetry experiment Chapter 7 145 Evaluation and outlook Summary 155 Samenvatting 157 Dankwoord 159 Levensloop 161 Chapter 1 Introduction 1.1 The formation of porous structures by thermally-induced phase-separation Porous polymer membranes can be prepared by different techniques: track-etching, stretching and phase-separation [1]. -

Commercial Air Conditioner Haier and Higher
SYJS-002 Commercial Air Conditioner Haier and Higher Selected Design and Installation for Haier Commercial Air Conditioners Haier Group 2001 Большая библиотека технической документации http://splitoff.ru/tehn-doc.html каталоги, инструкции, сервисные мануалы, схемы. CONTENTS Chapter One Basic theory of air conditioning------------------------------------------1 Section one Basic features of humid air---------------------------------------------------1 1-1 Basic features of humid air---------------------------------------------------------------- 1 1-2 Temperature-------------------------------------------------------------------------------- 2 1-3 Humidity------------------------------------------------------------------------------------ 3 1-4 Enthalpy ------------------------------------------------------------------------------------ 7 Section two Humid air I-d diagram-------------------------------------------------------- 8 2-1 Humid air I-d diagram----------------------------------------------------------------------8 2-2 Application of the I-d diagram----------------------------------------------------------- 11 Section three Environmental conditions--------------------------------------------------16 3-1 Meteorological data of major Chinese cities--------------------------------------------16 3-2 Physiologically required air quality and quantity--------------------------------------16 3-3 Harmful gas, substance and maximum allowable odorous gas and ventilation------ 17 3-4 Pleasant air temperature and humidity range-------------------------------------------19 -

Interpreting DSC Curves Part 1: Dynamic Measurements
1/2000 Information for users of METTLER TOLEDO thermal analysis systems Dear Customer, The year 2000 should prove to be extremely interesting for METTLER TOLEDO thermal analysis. We plan to expand the very successful STARe product line with the introduction of an exciting new instrument for dynamic mechanical analysis. And of course the current thermal analysis instruments have been undergoing continuous development. In this edition of UserCom, we are delighted to present the new DSC822e. 11 Interpreting DSC curves Contents Part 1: Dynamic measurements TA TIP – Interpreting DSC curves; The art of interpreting curves has yet to be integrated into commercially available com- puter programs. The interpretation of a DSC measurement curve is therefore still some- Part 1: Dynamic measurements thing you have to do yourself. It requires a considerable amount of experience in thermal analysis as well as a knowledge of the possible reactions that your particular sample can NEW in our sales program undergo. – DSC822e This article presents tips and information that should help you with the systematic inter- pretation of DSC curves. Applications Recognizing artifacts – The glass transition from the point of The first thing to do is to examine the curve for any obvious artifacts that could lead to a view of DSC measurements; possible misinterpretation of the results. Artifacts are effects that are not caused by the Part 2: Information for the character- ization of materials sample under investigation. Figure 1 shows examples of a number of such artifacts. They include: – Thermal values of fats: DSC analysis or dropping point determination? a) An abrupt change of the heat transfer between the sample and the pan: 1) Samples of irregular form can topple over in the pan. -

Evaporative Cooling in a Bose Gas
Utrecht University Debye Institute Nanophotonics Group Evaporative Cooling in a Bose Gas Author: Supervisors: C. Beulenkamp Prof. dr. P. van der Straten Jasper Smits June 17, 2015 Abstract A method of simulating the evaporative cooling of neutral atoms in a magnetic trap is presented. The method is based on kinetic theory and an assumption of ergodicity. An application of this method to the experimental conditions in the Utrecht BEC experiment is compared to measurements. The simulation is found to be accurate down to a temperature of 10 µK. Its predictive power makes it a valuable tool in optimizing the cooling process. Contents Contents 1 Introduction 1 2 Experimental setup and methods 2 2.1 Experimental setup . .2 2.2 Magnetic Trap . .3 2.3 Imaging setup . .4 2.3.1 Absorption imaging . .4 2.3.2 Phase Contrast Imaging . .4 2.4 Measuring the trap parameters . .5 3 Theory 6 3.1 The Boltzmann equation and ergodicity . .6 3.2 Loss processes . .7 3.3 Thermodynamic properties . .9 3.4 RF induced evaporation . 11 3.5 The shape of a truncated distribution . 12 3.6 Effect of gravity on the truncation energy. 13 4 Simulation 14 4.1 Discretization . 14 4.2 Method . 15 5 Experiments 16 5.1 Linear Ramps . 16 5.1.1 30 second ramp . 17 5.1.2 50 second ramp . 19 5.2 An optimized path in the harmonic regime . 21 i Contents 5.2.1 Cooling in the harmonic regime . 22 6 Discussion & Conclusion 23 7 Acknowledgments 24 ii Introduction 1 Introduction Evaporative cooling is the process of cooling through removal of high energy particles. -

Chapter 4. the Physical Transformations of Pure Substances
Chapter 4. The Physical transformations of pure substances 2011 Fall Semester Physical Chemistry 1 (CHM2201) Contents Phase Diagrams 4.1 The stabilities of phases 4.2 Phase boundaries 4.3 Three representative phase diagrams Thermodynamic aspects of phase transitions 4.4 The dependence of stability on the conditions 4.5 The location of phase boundaries 4.6 The Ehrenfest classification of phase transitions Phase diagrams 4.1 The stabilities of phases Key points 1. A phase is a form of matter that is uniform throughout in chemical composition and physical state 2. A phase transition is the spontaneous conversion of one phase into another 3. The thermodynamic analysis of phases is based on the fact that, at equilibrium, the chemical potential of a substance is the same throughout a sample 4.1 The stabilities of phases (a) The number of phases • Phase : a form of matter that is uniform throughout in chemical composition and physical state 1. Solid, liquid, and gas phases • The number of phases, P 1. P = 1 : A gas, a crystal of a substance, two fully miscible liquids 2. P = 2 : A slurry of ice and water CaCO3(s) → CaO(s) + CO2 (g) 3. There are two solid phases and one gaseous phase A single phase A dispersion solution 4.1 The stabilities of phases (b) Phase transitions • Phase transition : the spontaneous conversion of one phase into another phase • A phase transition occurs at a characteristic temperature for a given pressure • Below 0℃ the Gibbs energy decreases as liquid water change into ice • Above 0℃ the Gibbs energy decreases as ice