Chapter 8 Phase Diagrams
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Thermodynamics
TREATISE ON THERMODYNAMICS BY DR. MAX PLANCK PROFESSOR OF THEORETICAL PHYSICS IN THE UNIVERSITY OF BERLIN TRANSLATED WITH THE AUTHOR'S SANCTION BY ALEXANDER OGG, M.A., B.Sc., PH.D., F.INST.P. PROFESSOR OF PHYSICS, UNIVERSITY OF CAPETOWN, SOUTH AFRICA THIRD EDITION TRANSLATED FROM THE SEVENTH GERMAN EDITION DOVER PUBLICATIONS, INC. FROM THE PREFACE TO THE FIRST EDITION. THE oft-repeated requests either to publish my collected papers on Thermodynamics, or to work them up into a comprehensive treatise, first suggested the writing of this book. Although the first plan would have been the simpler, especially as I found no occasion to make any important changes in the line of thought of my original papers, yet I decided to rewrite the whole subject-matter, with the inten- tion of giving at greater length, and with more detail, certain general considerations and demonstrations too concisely expressed in these papers. My chief reason, however, was that an opportunity was thus offered of presenting the entire field of Thermodynamics from a uniform point of view. This, to be sure, deprives the work of the character of an original contribution to science, and stamps it rather as an introductory text-book on Thermodynamics for students who have taken elementary courses in Physics and Chemistry, and are familiar with the elements of the Differential and Integral Calculus. The numerical values in the examples, which have been worked as applications of the theory, have, almost all of them, been taken from the original papers; only a few, that have been determined by frequent measurement, have been " taken from the tables in Kohlrausch's Leitfaden der prak- tischen Physik." It should be emphasized, however, that the numbers used, notwithstanding the care taken, have not vii x PREFACE. -

Lecture Notes: BCS Theory of Superconductivity
Lecture Notes: BCS theory of superconductivity Prof. Rafael M. Fernandes Here we will discuss a new ground state of the interacting electron gas: the superconducting state. In this macroscopic quantum state, the electrons form coherent bound states called Cooper pairs, which dramatically change the macroscopic properties of the system, giving rise to perfect conductivity and perfect diamagnetism. We will mostly focus on conventional superconductors, where the Cooper pairs originate from a small attractive electron-electron interaction mediated by phonons. However, in the so- called unconventional superconductors - a topic of intense research in current solid state physics - the pairing can originate even from purely repulsive interactions. 1 Phenomenology Superconductivity was discovered by Kamerlingh-Onnes in 1911, when he was studying the transport properties of Hg (mercury) at low temperatures. He found that below the liquifying temperature of helium, at around 4:2 K, the resistivity of Hg would suddenly drop to zero. Although at the time there was not a well established model for the low-temperature behavior of transport in metals, the result was quite surprising, as the expectations were that the resistivity would either go to zero or diverge at T = 0, but not vanish at a finite temperature. In a metal the resistivity at low temperatures has a constant contribution from impurity scattering, a T 2 contribution from electron-electron scattering, and a T 5 contribution from phonon scattering. Thus, the vanishing of the resistivity at low temperatures is a clear indication of a new ground state. Another key property of the superconductor was discovered in 1933 by Meissner. -

Low Temperature Soldering Using Sn-Bi Alloys
LOW TEMPERATURE SOLDERING USING SN-BI ALLOYS Morgana Ribas, Ph.D., Anil Kumar, Divya Kosuri, Raghu R. Rangaraju, Pritha Choudhury, Ph.D., Suresh Telu, Ph.D., Siuli Sarkar, Ph.D. Alpha Assembly Solutions, Alpha Assembly Solutions India R&D Centre Bangalore, KA, India [email protected] ABSTRACT package substrate and PCB [2-4]. This represents a severe Low temperature solder alloys are preferred for the limitation on using the latest generation of ultra-thin assembly of temperature-sensitive components and microprocessors. Use of low temperature solders can substrates. The alloys in this category are required to reflow significantly reduce such warpage, but available Sn-Bi between 170 and 200oC soldering temperatures. Lower solders do not match Sn-Ag-Cu drop shock performance [5- soldering temperatures result in lower thermal stresses and 6]. Besides these pressing technical requirements, finding a defects, such as warping during assembly, and permit use of low temperature solder alloy that can replace alloys such as lower cost substrates. Sn-Bi alloys have lower melting Sn-3Ag-0.5Cu solder can result in considerable hard dollar temperatures, but some of its performance drawbacks can be savings from reduced energy cost and noteworthy reduction seen as deterrent for its use in electronics devices. Here we in carbon emissions [7]. show that non-eutectic Sn-Bi alloys can be used to improve these properties and further align them with the electronics In previous works [8-11] we have showed how the use of industry specific needs. The physical properties and drop micro-additives in eutectic Sn-Bi alloys results in significant shock performance of various alloys are evaluated, and their improvement of its thermo-mechanical properties. -

Supercritical Fluid Extraction of Positron-Emitting Radioisotopes from Solid Target Matrices
XA0101188 11. United States of America Supercritical Fluid Extraction of Positron-Emitting Radioisotopes From Solid Target Matrices D. Schlyer, Brookhaven National Laboratory, Chemistry Department, Upton, Bldg. 901, New York 11973-5000, USA Project Description Supercritical fluids are attractive as media for both chemical reactions, as well as process extraction since their physical properties can be manipulated by small changes in pressure and temperature near the critical point of the fluid. What is a supercritical fluid? Above a certain temperature, a vapor can no longer be liquefied regardless of pressure critical temperature - Tc supercritical fluid r«gi on solid a u & temperature Fig. 1. Phase diagram depicting regions of solid, liquid, gas and supercritical fluid behavior. The critical point is defined by a critical pressure (Pc) and critical temperature (Tc) for a particular substance. Such changes can result in drastic effects on density-dependent properties such as solubility, refractive index, dielectric constant, viscosity and diffusivity of the fluid[l,2,3]. This suggests that pressure tuning of a pure supercritical fluid may be a useful means to manipulate chemical reactions on the basis of a thermodynamic solvent effect. It also means that the solvation properties of the fluid can be precisely controlled to enable selective component extraction from a matrix. In recent years there has been a growing interest in applying supercritical fluid extraction to the selective removal of trace metals from solid samples [4-10]. Much of the work has been done on simple systems comprised of inert matrices such as silica or cellulose. Recently, this process as been expanded to environmental samples as well [11,12]. -

The Basics of Soldering
TECH SOLUTION: SOLDER The Basics of Soldering SUmmARY BY Chris Nash, INDIUM CORPORATION IN In this article, I will present a basic overview of soldering for those Soldering uses a filler metal and, in who are new to the world of most cases, an appropriate flux. The soldering and for those who could filler metals are typically alloys (al- though there are some pure metal sol- use a refresher. I will discuss the ders) that have liquidus temperatures below 350°C. Elemental metals com- definition of soldering, the basics monly alloyed in the filler metals or of metallurgy, how to choose the solders are tin, lead, antimony, bis- muth, indium, gold, silver, cadmium, proper alloy, the purpose of a zinc, and copper. By far, the most flux, soldering temperatures, and common solders are based on tin. Fluxes often contain rosin, acids (or- typical heating sources for soldering ganic or mineral), and/or halides, de- operations. pending on the desired flux strength. These ingredients reduce the oxides on the solder and mating pieces. hroughout history, as society has evolved, so has the need for bond- Basic Solder Metallurgy ing metals to metals. Whether the As heat is gradually applied to sol- need for bonding metals is mechani- der, the temperature rises until the Tcal, electrical, or thermal, it can be accom- alloy’s solidus point is reached. The plished by using solder. solidus point is the highest temper- ature at which an alloy is completely Metallurgical Bonding Processes solid. At temperatures just above Attachment of one metal to another can solidus, the solder is a mixture of be accomplished in three ways: welding, liquid and solid phases (analogous brazing, and soldering. -

Robust High-Temperature Heat Exchangers (Topic 2A Gen 3 CSP Project; DE-EE0008369)
Robust High-Temperature Heat Exchangers (Topic 2A Gen 3 CSP Project; DE-EE0008369) Illustrations of: (left) porous WC preform plates, (middle) dense-wall ZrC/W plates with horizontal channels and vertical vias. (Right) Backscattered electron image of the dense microstructure of a ZrC/W cermet. Team: Ken H. Sandhage1 (PI), Kevin P. Trumble1 (Co-PI), Asegun Henry2 (Co-PI), Aaron Wildberger3 (Co-PI) 1School of Materials Engineering, Purdue University, W. Lafayette, IN 2Department of Mechanical Engineering, Massachusetts Institute of Technology, Cambridge, MA 3Vacuum Process Engineering, Inc., Sacramento, CA Concentrated Solar Power Tower “Concentrating Solar Power Gen3 Demonstration Roadmap,” M. Mehos, C. Turchi, J. Vidal, M. Wagner, Z. Ma, C. Ho, W. Kolb, C. Andraka, A. Kruizenga, Technical Report NREL/TP-5500-67464, National Renewable Energy Laboratory, 2017 Concentrated Solar Power Tower Heat Exchanger “Concentrating Solar Power Gen3 Demonstration Roadmap,” M. Mehos, C. Turchi, J. Vidal, M. Wagner, Z. Ma, C. Ho, W. Kolb, C. Andraka, A. Kruizenga, Technical Report NREL/TP-5500-67464, National Renewable Energy Laboratory, 2017 State of the Art: Metal Alloy Printed Circuit HEXs Current Technology: • Printed Circuit HEXs: patterned etching of metallic alloy plates, then diffusion bonding • Metal alloy mechanical properties degrade significantly above 600oC D. Southall, S.J. Dewson, Proc. ICAPP '10, San Diego, CA, 2010; R. Le Pierres, et al., Proc. SCO2 Power Cycle Symposium 2011, Boulder, CO, 2011; D. Southall, et al., Proc. ICAPP '08, Anaheim, -
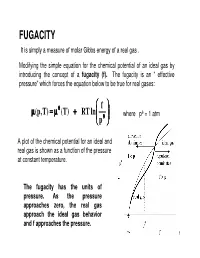
FUGACITY It Is Simply a Measure of Molar Gibbs Energy of a Real Gas
FUGACITY It is simply a measure of molar Gibbs energy of a real gas . Modifying the simple equation for the chemical potential of an ideal gas by introducing the concept of a fugacity (f). The fugacity is an “ effective pressure” which forces the equation below to be true for real gases: θθθ f µµµ ,p( T) === µµµ (T) +++ RT ln where pθ = 1 atm pθθθ A plot of the chemical potential for an ideal and real gas is shown as a function of the pressure at constant temperature. The fugacity has the units of pressure. As the pressure approaches zero, the real gas approach the ideal gas behavior and f approaches the pressure. 1 If fugacity is an “effective pressure” i.e, the pressure that gives the right value for the chemical potential of a real gas. So, the only way we can get a value for it and hence for µµµ is from the gas pressure. Thus we must find the relation between the effective pressure f and the measured pressure p. let f = φ p φ is defined as the fugacity coefficient. φφφ is the “fudge factor” that modifies the actual measured pressure to give the true chemical potential of the real gas. By introducing φ we have just put off finding f directly. Thus, now we have to find φ. Substituting for φφφ in the above equation gives: p µ=µ+(p,T)θ (T) RT ln + RT ln φ=µ (ideal gas) + RT ln φ pθ µµµ(p,T) −−− µµµ(ideal gas ) === RT ln φφφ This equation shows that the difference in chemical potential between the real and ideal gas lies in the term RT ln φφφ.φ This is the term due to molecular interaction effects. -

Equation of State and Phase Transitions in the Nuclear
National Academy of Sciences of Ukraine Bogolyubov Institute for Theoretical Physics Has the rights of a manuscript Bugaev Kyrill Alekseevich UDC: 532.51; 533.77; 539.125/126; 544.586.6 Equation of State and Phase Transitions in the Nuclear and Hadronic Systems Speciality 01.04.02 - theoretical physics DISSERTATION to receive a scientific degree of the Doctor of Science in physics and mathematics arXiv:1012.3400v1 [nucl-th] 15 Dec 2010 Kiev - 2009 2 Abstract An investigation of strongly interacting matter equation of state remains one of the major tasks of modern high energy nuclear physics for almost a quarter of century. The present work is my doctor of science thesis which contains my contribution (42 works) to this field made between 1993 and 2008. Inhere I mainly discuss the common physical and mathematical features of several exactly solvable statistical models which describe the nuclear liquid-gas phase transition and the deconfinement phase transition. Luckily, in some cases it was possible to rigorously extend the solutions found in thermodynamic limit to finite volumes and to formulate the finite volume analogs of phases directly from the grand canonical partition. It turns out that finite volume (surface) of a system generates also the temporal constraints, i.e. the finite formation/decay time of possible states in this finite system. Among other results I would like to mention the calculation of upper and lower bounds for the surface entropy of physical clusters within the Hills and Dales model; evaluation of the second virial coefficient which accounts for the Lorentz contraction of the hard core repulsing potential between hadrons; inclusion of large width of heavy quark-gluon bags into statistical description. -

Lecture 15: 11.02.05 Phase Changes and Phase Diagrams of Single- Component Materials
3.012 Fundamentals of Materials Science Fall 2005 Lecture 15: 11.02.05 Phase changes and phase diagrams of single- component materials Figure removed for copyright reasons. Source: Abstract of Wang, Xiaofei, Sandro Scandolo, and Roberto Car. "Carbon Phase Diagram from Ab Initio Molecular Dynamics." Physical Review Letters 95 (2005): 185701. Today: LAST TIME .........................................................................................................................................................................................2� BEHAVIOR OF THE CHEMICAL POTENTIAL/MOLAR FREE ENERGY IN SINGLE-COMPONENT MATERIALS........................................4� The free energy at phase transitions...........................................................................................................................................4� PHASES AND PHASE DIAGRAMS SINGLE-COMPONENT MATERIALS .................................................................................................6� Phases of single-component materials .......................................................................................................................................6� Phase diagrams of single-component materials ........................................................................................................................6� The Gibbs Phase Rule..................................................................................................................................................................7� Constraints on the shape of -

A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene-Ethylbenzene
Loyola University Chicago Loyola eCommons Master's Theses Theses and Dissertations 1940 A Study of the Equilibrium Diagrams of the Systems, Benzene- Toluene and Benzene-Ethylbenzene John B. Mullen Loyola University Chicago Follow this and additional works at: https://ecommons.luc.edu/luc_theses Part of the Chemistry Commons Recommended Citation Mullen, John B., "A Study of the Equilibrium Diagrams of the Systems, Benzene-Toluene and Benzene- Ethylbenzene" (1940). Master's Theses. 299. https://ecommons.luc.edu/luc_theses/299 This Thesis is brought to you for free and open access by the Theses and Dissertations at Loyola eCommons. It has been accepted for inclusion in Master's Theses by an authorized administrator of Loyola eCommons. For more information, please contact [email protected]. This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License. Copyright © 1940 John B. Mullen A S'l'IJDY OF THE EQUTI.IBRIUM DIAGRAMS OF THE SYSTEMS, BENZENE-TOLUENE AND BENZENE-~ENZENE By John B. Mullen Presented in partial :f'u.J.tilment at the requirements :f'or the degree o:f' Master o:f' Science, Loyola University, 1940. T.ABLE OF CONTENTS Page Acknowledgment 1i Vita iii Introduction 1 I. Review of Literature 2 II. Apparatus and its Calibration 3 III. Procedure am Technique 6 IV. Observations on the System Benzene-Toluene 9 V. Observations on the System Benzene-Ethylbenzene 19 VI. Equilibrium Diagrams of the Systems 30 Recommendations for Future Work 34 Bibliography 35 ACKNOWLEOOMENT The au1hor wishes to acknowledge with thanks the invaluable assistance, suggestions, and cooperation offered by Dr. -

Phase Diagrams
Module-07 Phase Diagrams Contents 1) Equilibrium phase diagrams, Particle strengthening by precipitation and precipitation reactions 2) Kinetics of nucleation and growth 3) The iron-carbon system, phase transformations 4) Transformation rate effects and TTT diagrams, Microstructure and property changes in iron- carbon system Mixtures – Solutions – Phases Almost all materials have more than one phase in them. Thus engineering materials attain their special properties. Macroscopic basic unit of a material is called component. It refers to a independent chemical species. The components of a system may be elements, ions or compounds. A phase can be defined as a homogeneous portion of a system that has uniform physical and chemical characteristics i.e. it is a physically distinct from other phases, chemically homogeneous and mechanically separable portion of a system. A component can exist in many phases. E.g.: Water exists as ice, liquid water, and water vapor. Carbon exists as graphite and diamond. Mixtures – Solutions – Phases (contd…) When two phases are present in a system, it is not necessary that there be a difference in both physical and chemical properties; a disparity in one or the other set of properties is sufficient. A solution (liquid or solid) is phase with more than one component; a mixture is a material with more than one phase. Solute (minor component of two in a solution) does not change the structural pattern of the solvent, and the composition of any solution can be varied. In mixtures, there are different phases, each with its own atomic arrangement. It is possible to have a mixture of two different solutions! Gibbs phase rule In a system under a set of conditions, number of phases (P) exist can be related to the number of components (C) and degrees of freedom (F) by Gibbs phase rule. -

Phase Transitions in Multicomponent Systems
Physics 127b: Statistical Mechanics Phase Transitions in Multicomponent Systems The Gibbs Phase Rule Consider a system with n components (different types of molecules) with r phases in equilibrium. The state of each phase is defined by P,T and then (n − 1) concentration variables in each phase. The phase equilibrium at given P,T is defined by the equality of n chemical potentials between the r phases. Thus there are n(r − 1) constraints on (n − 1)r + 2 variables. This gives the Gibbs phase rule for the number of degrees of freedom f f = 2 + n − r A Simple Model of a Binary Mixture Consider a condensed phase (liquid or solid). As an estimate of the coordination number (number of nearest neighbors) think of a cubic arrangement in d dimensions giving a coordination number 2d. Suppose there are a total of N molecules, with fraction xB of type B and xA = 1 − xB of type A. In the mixture we assume a completely random arrangement of A and B. We just consider “bond” contributions to the internal energy U, given by εAA for A − A nearest neighbors, εBB for B − B nearest neighbors, and εAB for A − B nearest neighbors. We neglect other contributions to the internal energy (or suppose them unchanged between phases, etc.). Simple counting gives the internal energy of the mixture 2 2 U = Nd(xAεAA + 2xAxBεAB + xBεBB) = Nd{εAA(1 − xB) + εBBxB + [εAB − (εAA + εBB)/2]2xB(1 − xB)} The first two terms in the second expression are just the internal energy of the unmixed A and B, and so the second term, depending on εmix = εAB − (εAA + εBB)/2 can be though of as the energy of mixing.