Two Cases with CD-20 Negative Diffuse Large B-Cell Lymphoma and Literature Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Primary Mediastinal Large B-Cell Lymphoma
Primary Mediastinal Large B-Cell Lymphoma Page 1 of 13 Disclaimer: This algorithm has been developed for MD Anderson using a multidisciplinary approach considering circumstances particular to MD Anderson’s specific patient population, services and structure, and clinical information. This is not intended to replace the independent medical or professional judgment of physicians or other health care providers in the context of individual clinical circumstances to determine a patient's care. This algorithm should not be used to treat pregnant women. Note: Consider Clinical Trials as treatment options for eligible patients. PATHOLOGIC DIAGNOSIS INITIAL EVALUATION ESSENTIAL: 5 ● Physical exam: attention to node-bearing areas, including Waldeyer's ring, ESSENTIAL: and to size of liver and spleen ● ECOG performance status ● Hematopathology review of all slides with at least one paraffin ● B symptoms (Unexplained fever >38°C during the previous month; block or 15 unstained slides representative of the tumor. Rebiopsy if Recurrent drenching night sweats during the previous month; Weight consult material is nondiagnostic. loss >10 percent of body weight ≤ 6 months of diagnosis) ● Adequate morphology and immunophenotyping to establish 1 ● CBC with differential, LDH, BUN, creatinine, albumin, AST, diagnosis ALT, total bilirubin, alkaline phosphatase, serum calcium, uric acid ○ Paraffin Panel: CD3, CD20 and/or another pan-B-cell marker ● Beta 2 microglobulin (CD19, PAX-5, CD79a) or ● Screening for HIV 1 and 2, hepatitis B and C (HBcAb, HBsAg, HCV -

Ilite™ ADCC Target CD20 (-) Assay Ready Cells (REF: BM4015)
PRODUCT SPECIFICATION iLite™ ADCC Target CD20 (-) Assay Ready Cells (REF: BM4015) Description iLite™ ADCC Target CD20 (-) Assay Ready Cells are based on a human B lymphocyte cell line, Raji (ATCC# CCL-86), and have been genetically engineered and optimized to be depleted of CD20 expression. The cells are to be use as negative controls together with iLite™ ADCC Effector (V) Assay Ready Cells and iLite™ ADCC Target CD20 (+) Assay Ready Cells for measuring the ADCC activity of anti-CD20 antibodies. Content >250 µL of iLite™ Assay Ready Cells suspended in RPMI 1640 with 20% FBS, mixed 1:1 with cryoprotective medium from Lonza (Cat. No 12-132A). Receipt and storage Upon receipt confirm that adequate dry-ice is present and the cells are frozen. Immediately transfer to -80°C storage. Cells should be stored at -80°C (do not store at any other temperature) and are stable as supplied until the expiry date shown. Cells should be used within 30 min of thawing. Background Antibody-dependent cell-mediated cytotoxicity (ADCC) is a mechanism whereby pathogenic cells are lysed by lymphocytes, most often Natural Killer (NK) cells. The mechanism involves binding of antibodies to surface antigens on the pathogen. Crosslinking of these antibodies to NK cells through the binding of the Fc-portion to Fc receptors on the NK cells leads to activation of the NK cell and formation of an immune synapse with the pathogenic cell. The NK cell releases cytotoxic granules containing granzymes and perforin into the synapse, leading to apoptosis of the targeted cell (1). The idea of employing ADCC to destroy dysfunctional cells by treating patients with antibodies has existed since the discovery of the ADCC mechanism. -

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’S Lymphomas Version 2.2015
NCCN Guidelines Index NHL Table of Contents Discussion NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’s Lymphomas Version 2.2015 NCCN.org Continue Version 2.2015, 03/03/15 © National Comprehensive Cancer Network, Inc. 2015, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN® . Peripheral T-Cell Lymphomas NCCN Guidelines Version 2.2015 NCCN Guidelines Index NHL Table of Contents Peripheral T-Cell Lymphomas Discussion DIAGNOSIS SUBTYPES ESSENTIAL: · Review of all slides with at least one paraffin block representative of the tumor should be done by a hematopathologist with expertise in the diagnosis of PTCL. Rebiopsy if consult material is nondiagnostic. · An FNA alone is not sufficient for the initial diagnosis of peripheral T-cell lymphoma. Subtypes included: · Adequate immunophenotyping to establish diagnosisa,b · Peripheral T-cell lymphoma (PTCL), NOS > IHC panel: CD20, CD3, CD10, BCL6, Ki-67, CD5, CD30, CD2, · Angioimmunoblastic T-cell lymphoma (AITL)d See Workup CD4, CD8, CD7, CD56, CD57 CD21, CD23, EBER-ISH, ALK · Anaplastic large cell lymphoma (ALCL), ALK positive (TCEL-2) or · ALCL, ALK negative > Cell surface marker analysis by flow cytometry: · Enteropathy-associated T-cell lymphoma (EATL) kappa/lambda, CD45, CD3, CD5, CD19, CD10, CD20, CD30, CD4, CD8, CD7, CD2; TCRαβ; TCRγ Subtypesnot included: · Primary cutaneous ALCL USEFUL UNDER CERTAIN CIRCUMSTANCES: · All other T-cell lymphomas · Molecular analysis to detect: antigen receptor gene rearrangements; t(2;5) and variants · Additional immunohistochemical studies to establish Extranodal NK/T-cell lymphoma, nasal type (See NKTL-1) lymphoma subtype:βγ F1, TCR-C M1, CD279/PD1, CXCL-13 · Cytogenetics to establish clonality · Assessment of HTLV-1c serology in at-risk populations. -

Drug Resistance in Non-Hodgkin Lymphomas
International Journal of Molecular Sciences Review Drug Resistance in Non-Hodgkin Lymphomas Pavel Klener 1,2,* and Magdalena Klanova 1,2 1 First Department of Internale Medicine-Hematology, University General Hospital in Prague, 128 08 Prague, Czech Republic; [email protected] 2 Institute of Pathological Physiology, First Faculty of Medicine, Charles University, Prague, 128 53 Prague, Czech Republic * Correspondence: [email protected] or [email protected] Received: 3 February 2020; Accepted: 15 March 2020; Published: 18 March 2020 Abstract: Non-Hodgkin lymphomas (NHL) are lymphoid tumors that arise by a complex process of malignant transformation of mature lymphocytes during various stages of differentiation. The WHO classification of NHL recognizes more than 90 nosological units with peculiar pathophysiology and prognosis. Since the end of the 20th century, our increasing knowledge of the molecular biology of lymphoma subtypes led to the identification of novel druggable targets and subsequent testing and clinical approval of novel anti-lymphoma agents, which translated into significant improvement of patients’ outcome. Despite immense progress, our effort to control or even eradicate malignant lymphoma clones has been frequently hampered by the development of drug resistance with ensuing unmet medical need to cope with relapsed or treatment-refractory disease. A better understanding of the molecular mechanisms that underlie inherent or acquired drug resistance might lead to the design of more effective front-line treatment algorithms based on reliable predictive markers or personalized salvage therapy, tailored to overcome resistant clones, by targeting weak spots of lymphoma cells resistant to previous line(s) of therapy. This review focuses on the history and recent advances in our understanding of molecular mechanisms of resistance to genotoxic and targeted agents used in clinical practice for the therapy of NHL. -

REVIEW Anti-CD20-Based Therapy of B Cell Lymphoma: State of The
Leukemia (2002) 16, 2004–2015 2002 Nature Publishing Group All rights reserved 0887-6924/02 $25.00 www.nature.com/leu REVIEW Anti-CD20-based therapy of B cell lymphoma: state of the art C Kosmas1, K Stamatopoulos2, N Stavroyianni2, N Tsavaris3 and T Papadaki4 1Department of Medicine, 2nd Division of Medical Oncology, ‘Metaxa’ Cancer Hospital, Piraeus, Greece; 2Department of Hematology, G Papanicolaou General Hospital, Thessaloniki, Greece; 3Oncology Unit, Department of Pathophysiology, Athens University School of Medicine, Laikon General Hospital, Athens, Greece; and 4Hemopathology Department, Evangelismos Hospital, Athens, Greece Over the last 5 years, studies applying the chimeric anti-CD20 ficulties in identifying a completely tumor-specific target; (2) MAb have renewed enthusiasm and triggered world-wide appli- the impracticality of constructing a unique antibody for each cation of anti-CD20 MAb-based therapies in B cell non-Hodg- kin’s lymphoma (NHL). Native chimeric anti-CD20 and isotope- patient; (3) the development of an immune response to murine 6 labeled murine anti-CD20 MAbs are currently employed with immunoglobulins (human anti-mouse antibodies, HAMA). By encouraging results as monotherapy or in combination with the end of the 1980s enthusiasm for therapeutic MAbs was conventional chemotherapy and in consolidation of remission waning; murine native (unconjugated), radioactively labeled after treatments with curative intent (ie after/ in combination or toxin-conjugated MAbs failed to yield significant clinical with high-dose chemotherapy and hematopoietic stem cell responses; moreover, they were not uncommonly associated rescue). On the available experience, anti-CD20 MAb-based therapeutic strategies will be increasingly integrated in the with toxicities, predominantly in the form of serum sickness treatment of B cell NHL and related malignancies. -

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Myeloid Growth Factors
NCCN Guidelines Index MGF Table of Contents Discussion NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Myeloid Growth Factors Version 2.2013 NCCN.org Continue Version 2.2013, 08/02/13 © National Comprehensive Cancer Network, Inc. 2013, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®. NCCN Guidelines Version 2.2013 Panel Members NCCN Guidelines Index MGF Table of Contents Myeloid Growth Factors Discussion * Jeffrey Crawford, MD/Chair †‡ Susan Hudock, PharmD å Lee S. Schwartzberg, MD † ‡ Þ Duke Cancer Institute The Sidney Kimmel Comprehensive St. Jude Children's Research Hospital/ Cancer Center at Johns Hopkins The University of Tennessee Health Science James Armitage, MD † x Center- The West Clinic UNMC Eppley Cancer Center at Dwight D. Kloth, PharmD å å The Nebraska Medical Center Fox Chase Cancer Center Sepideh Shayani, PharmD City of Hope Comprehensive Cancer Center Lodovico Balducci, MD † ‡ David J. Kuter, MD, DPhil † ‡ Moffitt Cancer Center Massachusetts General David P. Steensma, MD†‡Þ Hospital Cancer Center Dana-Farber/Brigham and Women’s Pamela Sue Becker, MD, PhD ‡ Þ x Cancer Center Fred Hutchinson Cancer Research Center/ Gary H. Lyman, MD, MPH † ‡ å Seattle Cancer Care Alliance Duke Cancer Institute Mahsa Talbott, PharmD Vanderbilt-Ingram Cancer Center Douglas W. Blayney, MD † Brandon McMahon, MD ‡ Stanford Cancer Institute Robert H. Lurie Comprehensive Cancer Saroj Vadhan-Raj, MD † Þ Center of Northwestern University The University of Texas Spero R. Cataland, MD ‡ MD Anderson Cancer Center The Ohio State University Comprehensive Hope S. Rugo, MD † ‡ x Cancer Center - James Cancer Hospital UCSF Helen Diller Family Peter Westervelt, MD, PhD † Siteman Cancer Center at Barnes- and Solove Research Institute Comprehensive Cancer Center Jewish Hospital and Washington University School of Medicine Mark L. -
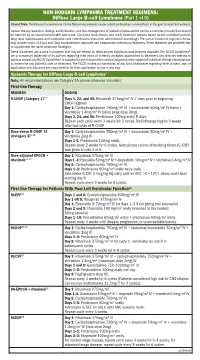
Diffuse Large B-Cell Lymphoma (Part 1 of 5)
NON-HODGKIN LYMPHOMA TREATMENT REGIMENS: Diffuse Large B-cell Lymphoma (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Systemic Therapy for Diffuse Large B-cell Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. First-line Therapy REGIMEN DOSING R-CHOP (Category 1)2–7 Days 1, 22, and 43: Rituximab 375mg/m2 IV 7 days prior to beginning CHOP regimen Day 1: Cyclophosphamide 750mg/m2 IV + doxorubicin 50mg/m2 IV bolus + vincristine 1.4mg/m2 IV bolus (max dose 2mg) Days 3, 24, and 45: Prednisone 100mg orally 5 days. -

Poor Mobilization Is an Independent Prognostic Factor in Patients with Malignant Lymphomas Treated by Peripheral Blood Stem Cell Transplantation
Bone Marrow Transplantation (2006) 37, 719–724 & 2006 Nature Publishing Group All rights reserved 0268-3369/06 $30.00 www.nature.com/bmt ORIGINAL ARTICLE Poor mobilization is an independent prognostic factor in patients with malignant lymphomas treated by peripheral blood stem cell transplantation V Pavone1,2, F Gaudio1, G Console3, U Vitolo4, P Iacopino3, A Guarini1, V Liso1, T Perrone1 and A Liso5 1Hematology Department, University of Bari, Bari, Italy; 2Hematology Department, Hospital ‘C Panico’, Tricase, Italy; 3Bone Marrow Transplantation Unit, Reggio Calabria, Italy; 4Haematology Department, Turin Hospital, Turin, Italy and 5Hematology Unit, University of Foggia, Foggia, Italy Haemopoietic stem cell therapy is an increasingly adopted ment frequently employed in relapsed malignant lympho- procedure in the treatment of patients with malignant mas (ML) or in very high-risk ML.2–12 The presence of lymphoma. In this retrospective analysis, we evaluated HSCs in peripheral blood is usually extremely low before 262 patients, 57 (22%) with Hodgkin’s and 205 (78%) mobilizing procedures, and engraftment of CD34 þ per- with non-Hodgkin’s lymphomas (NHL), and 665 harvest- ipheral blood stem cells (PBSC) depends on the infusion of ing procedures in order to assess the impact of poor an adequate number of CD34 þ stem cells to restore mobilization on survival and to determine the factors that haemopoiesis.13–23 Indeed, the number of CD34 þ cells is may be predictive of CD34 þ poor mobilization. The commonly used to predict the potential engraftment of mobilization chemotherapy regimens consisted of high- harvested HSC.17,19,22,23 A cutoff of 20CD34 þ cells/mlin dose cyclophosphamide in 92 patients (35.1%) and a high- the peripheral blood has been arbitrarily defined to predict dose cytarabine-containing regimen (DHAP in 87 patients a successful collection procedure, and an infusion of a –(33.2%), MAD in 83 (31.7%)). -

Targeted Drugs As Maintenance Therapy After Autologous Stem Cell Transplantation in Patients with Mantle Cell Lymphoma
pharmaceuticals Review Targeted Drugs as Maintenance Therapy after Autologous Stem Cell Transplantation in Patients with Mantle Cell Lymphoma Fengting Yan 1,2, Ajay K. Gopal 1,2 and Solomon A. Graf 1,2,3,* 1 Department of Medicine, University of Washington, Seattle, WA 98195, USA; [email protected] (F.Y.); [email protected] (A.K.G.) 2 Fred Hutchinson Cancer Research Center, Seattle, WA 98109, USA 3 Veterans Affairs Puget Sound Health Care System, Seattle, WA 98108, USA * Correspondence: [email protected]; Tel.: +01-206-277-4757 Academic Editor: Luciano J. Costa Received: 27 January 2017; Accepted: 8 March 2017; Published: 10 March 2017 Abstract: The treatment landscape for mantle cell lymphoma (MCL) is rapidly evolving toward the incorporation of novel and biologically targeted pharmaceuticals with improved disease activity and gentler toxicity profiles compared with conventional chemotherapeutics. Upfront intensive treatment of MCL includes autologous stem cell transplantation (SCT) consolidation aimed at deepening and lengthening disease remission, but subsequent relapse occurs. Maintenance therapy after autologous SCT in patients with MCL in remission features lower-intensity treatments given over extended periods to improve disease outcomes. Targeted drugs are a natural fit for this space, and are the focus of considerable clinical investigation. This review summarizes recent advances in the field and their potential impact on treatment practices for MCL. Keywords: stem cell transplantation; mantle cell lymphoma; maintenance therapy 1. Introduction Mantle cell lymphoma (MCL) is an uncommon and heterogeneous subtype of B-cell non-Hodgkin lymphoma (B-NHL). It arises from antigen-naïve B-cells that proliferate in the mantle zone of lymph node germinal centers, and typically presents in an advanced stage, involving lymph nodes and extranodal sites including the gastrointestinal tract. -

The Role of Glucocorticoids in the Treatment of Non-Hodgkin Lymphoma
Open Access Annals of Hematology & Oncology Review Article The Role of Glucocorticoids in the Treatment of Non- Hodgkin Lymphoma Lamar ZS1,2* 1Department of Internal Medicine, Section on Abstract Hematology and Oncology, Wake Forest School of First line chemotherapy for aggressive non-Hodgkin lymphoma (NHL) Medicine, USA typically involves high doses of glucocorticoids (GCs) over several days. The 2Comprehensive Cancer Center, Wake Forest Baptist most commonly used combination chemotherapy regimen for NHL includes Medical Center, Winston Salem, USA cyclophosphamide, adriamycin, vincristine, and prednisone (CHOP) or given *Corresponding author: Zanetta S. Lamar, with rituximab (R-CHOP). The dose of prednisone used in the R-CHOP Department of Internal Medicine, Section on Hematology regimen varies in historical studies and in current clinical trials. There is a and Oncology, Wake Forest School of Medicine, Medical paucity of prospective data outlining the management of hyperglycemia during Center Blvd, Winston Salem, NC 27157, USA chemotherapy in diabetics or the risk of hyperglycemia or steroid-induced hyperglycemia during or following chemotherapy. Often, the adverse short and Received: June 18, 2016; Accepted: August 22, 2016; long-term effects of high doses of GCs are not reported in clinical trials. We Published: August 24, 2016 will discuss the history of GC incorporation into combination chemotherapy for lymphoma, the potential implications of liberal GC use in this population, and the opportunities for further research. Keywords: Glucocorticoid; Steroid; Non-Hodgkin lymphoma; Diabetes; Cancer; Hyperglycemia Introduction apoptosis is dependent on adequate levels of the GCR, the mechanism of GC-induced apoptosis is complex and involves multiple signaling Glucocorticoids (GCs) are a class of steroid hormones produced pathways [10,11]. -

A Case of Anaplastic Large Cell Lymphoma Presenting in Leukemic Phase
isord D ers od & lo T r B f a n o s l f a u n s r Journal of i o u n o J ISSN: 2155-9864 Ravilla et al., J Blood Disord Transfus 2015, 6:5 Blood Disorders & Transfusion DOI: 10.4172/2155-9864.1000316 Case Report Open Access A Case of Anaplastic Large Cell Lymphoma Presenting in Leukemic Phase Rahul Ravilla1*, Appalanaidu Sasapu1, Jeanette M Ramos2 and Konstantinos Arnaoutakis1 1Department of Hematology-Oncology, University of Arkansas for Medical Sciences, Little Rock, AR, USA 2Department of Pathology, University of Arkansas for Medical Sciences, Little Rock, AR, USA *Corresponding author: Rahul Ravilla, Department of Hematology-Oncology, University of Arkansas for Medical Sciences, Little Rock, 4301 W. Markham St., Slot #634, Arkansas-72205, AR, USA, Tel: 501-313-9405; Fax: 501-686-6001; E-mail: [email protected] Received date: Sep 22, 2015, Accepted date: Oct 24, 2015, Publication date: Oct 31, 2015 Copyright: © 2015 Ravilla R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Anaplastic lymphoma kinase (ALK) with positive Anaplastic Large Cell Lymphoma (ALCL) is has a distinct entity among the peripheral T-cell lymphomas. With a 5-year survival rate of 70%, it carries one of the best prognoses among peripheral T cell lymphomas. In rare instances, ALK positive ALCL presents in leukemic phase and it has a very poor prognosis with negligible number of cases reporting a survival rate of beyond one year. -

Modified DHAP Regimen in the Salvage Treatment of Refractory Or
Journal of Cancer Research and Clinical Oncology (2019) 145:3067–3073 https://doi.org/10.1007/s00432-019-03027-6 ORIGINAL ARTICLE – CLINICAL ONCOLOGY Modifed DHAP regimen in the salvage treatment of refractory or relapsed lymphomas Frank Kroschinsky1 · Denise Röllig1 · Barbara Riemer1 · Michael Kramer1 · Rainer Ordemann1 · Johannes Schetelig1 · Martin Bornhäuser1 · Gerhard Ehninger1 · Mathias Hänel2 Received: 6 June 2019 / Accepted: 16 September 2019 / Published online: 28 September 2019 © Springer-Verlag GmbH Germany, part of Springer Nature 2019 Abstract Background The combination of dexamethasone, high-dose cytarabine, and cisplatin (DHAP) is an established salvage regi- men for lymphoma patients. We hypothesized that a modifed administration schedule for cisplatin and cytarabine results in lower toxicity and improved efcacy. Methods We retrospectively analysed 119 patients with relapsed or refractory, aggressive, or indolent B-cell lymphomas, mantle-cell lymphomas, peripheral T-cell lymphomas, or Hodgkin’s lymphomas who were treated with the modifed DHAP (mDHAP) regimen (dexamethasone 40 mg 15 min-i.v. infusion, days 1–4; cytarabine 2 × 0.5 g/m2 1 h-i.v. infusion, days 1–4; cisplatin 25 mg/m2 24 h-i.v. infusion, days 1–4). Responding and eligible patients underwent stem-cell transplantation. Results In total, 185 treatment cycles were evaluable. Severe myelosuppression was the main toxicity occurring in 90% of the cycles. Febrile neutropenia or documented infection was found in less than 40%. Two patients died related to treatment (TRM, 1.7%). Nephrotoxicity did not exceed CTC grade 3, which occurred in four cycles only (2.2%). Complete (CR) or partial (PR) responses after mDHAP were documented in 16% and 39% (overall response rate 55%).