A Case of Anaplastic Large Cell Lymphoma Presenting in Leukemic Phase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ilite™ ADCC Target CD20 (-) Assay Ready Cells (REF: BM4015)
PRODUCT SPECIFICATION iLite™ ADCC Target CD20 (-) Assay Ready Cells (REF: BM4015) Description iLite™ ADCC Target CD20 (-) Assay Ready Cells are based on a human B lymphocyte cell line, Raji (ATCC# CCL-86), and have been genetically engineered and optimized to be depleted of CD20 expression. The cells are to be use as negative controls together with iLite™ ADCC Effector (V) Assay Ready Cells and iLite™ ADCC Target CD20 (+) Assay Ready Cells for measuring the ADCC activity of anti-CD20 antibodies. Content >250 µL of iLite™ Assay Ready Cells suspended in RPMI 1640 with 20% FBS, mixed 1:1 with cryoprotective medium from Lonza (Cat. No 12-132A). Receipt and storage Upon receipt confirm that adequate dry-ice is present and the cells are frozen. Immediately transfer to -80°C storage. Cells should be stored at -80°C (do not store at any other temperature) and are stable as supplied until the expiry date shown. Cells should be used within 30 min of thawing. Background Antibody-dependent cell-mediated cytotoxicity (ADCC) is a mechanism whereby pathogenic cells are lysed by lymphocytes, most often Natural Killer (NK) cells. The mechanism involves binding of antibodies to surface antigens on the pathogen. Crosslinking of these antibodies to NK cells through the binding of the Fc-portion to Fc receptors on the NK cells leads to activation of the NK cell and formation of an immune synapse with the pathogenic cell. The NK cell releases cytotoxic granules containing granzymes and perforin into the synapse, leading to apoptosis of the targeted cell (1). The idea of employing ADCC to destroy dysfunctional cells by treating patients with antibodies has existed since the discovery of the ADCC mechanism. -

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’S Lymphomas Version 2.2015
NCCN Guidelines Index NHL Table of Contents Discussion NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Non-Hodgkin’s Lymphomas Version 2.2015 NCCN.org Continue Version 2.2015, 03/03/15 © National Comprehensive Cancer Network, Inc. 2015, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN® . Peripheral T-Cell Lymphomas NCCN Guidelines Version 2.2015 NCCN Guidelines Index NHL Table of Contents Peripheral T-Cell Lymphomas Discussion DIAGNOSIS SUBTYPES ESSENTIAL: · Review of all slides with at least one paraffin block representative of the tumor should be done by a hematopathologist with expertise in the diagnosis of PTCL. Rebiopsy if consult material is nondiagnostic. · An FNA alone is not sufficient for the initial diagnosis of peripheral T-cell lymphoma. Subtypes included: · Adequate immunophenotyping to establish diagnosisa,b · Peripheral T-cell lymphoma (PTCL), NOS > IHC panel: CD20, CD3, CD10, BCL6, Ki-67, CD5, CD30, CD2, · Angioimmunoblastic T-cell lymphoma (AITL)d See Workup CD4, CD8, CD7, CD56, CD57 CD21, CD23, EBER-ISH, ALK · Anaplastic large cell lymphoma (ALCL), ALK positive (TCEL-2) or · ALCL, ALK negative > Cell surface marker analysis by flow cytometry: · Enteropathy-associated T-cell lymphoma (EATL) kappa/lambda, CD45, CD3, CD5, CD19, CD10, CD20, CD30, CD4, CD8, CD7, CD2; TCRαβ; TCRγ Subtypesnot included: · Primary cutaneous ALCL USEFUL UNDER CERTAIN CIRCUMSTANCES: · All other T-cell lymphomas · Molecular analysis to detect: antigen receptor gene rearrangements; t(2;5) and variants · Additional immunohistochemical studies to establish Extranodal NK/T-cell lymphoma, nasal type (See NKTL-1) lymphoma subtype:βγ F1, TCR-C M1, CD279/PD1, CXCL-13 · Cytogenetics to establish clonality · Assessment of HTLV-1c serology in at-risk populations. -

REVIEW Anti-CD20-Based Therapy of B Cell Lymphoma: State of The
Leukemia (2002) 16, 2004–2015 2002 Nature Publishing Group All rights reserved 0887-6924/02 $25.00 www.nature.com/leu REVIEW Anti-CD20-based therapy of B cell lymphoma: state of the art C Kosmas1, K Stamatopoulos2, N Stavroyianni2, N Tsavaris3 and T Papadaki4 1Department of Medicine, 2nd Division of Medical Oncology, ‘Metaxa’ Cancer Hospital, Piraeus, Greece; 2Department of Hematology, G Papanicolaou General Hospital, Thessaloniki, Greece; 3Oncology Unit, Department of Pathophysiology, Athens University School of Medicine, Laikon General Hospital, Athens, Greece; and 4Hemopathology Department, Evangelismos Hospital, Athens, Greece Over the last 5 years, studies applying the chimeric anti-CD20 ficulties in identifying a completely tumor-specific target; (2) MAb have renewed enthusiasm and triggered world-wide appli- the impracticality of constructing a unique antibody for each cation of anti-CD20 MAb-based therapies in B cell non-Hodg- kin’s lymphoma (NHL). Native chimeric anti-CD20 and isotope- patient; (3) the development of an immune response to murine 6 labeled murine anti-CD20 MAbs are currently employed with immunoglobulins (human anti-mouse antibodies, HAMA). By encouraging results as monotherapy or in combination with the end of the 1980s enthusiasm for therapeutic MAbs was conventional chemotherapy and in consolidation of remission waning; murine native (unconjugated), radioactively labeled after treatments with curative intent (ie after/ in combination or toxin-conjugated MAbs failed to yield significant clinical with high-dose chemotherapy and hematopoietic stem cell responses; moreover, they were not uncommonly associated rescue). On the available experience, anti-CD20 MAb-based therapeutic strategies will be increasingly integrated in the with toxicities, predominantly in the form of serum sickness treatment of B cell NHL and related malignancies. -

NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Myeloid Growth Factors
NCCN Guidelines Index MGF Table of Contents Discussion NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines® ) Myeloid Growth Factors Version 2.2013 NCCN.org Continue Version 2.2013, 08/02/13 © National Comprehensive Cancer Network, Inc. 2013, All rights reserved. The NCCN Guidelines® and this illustration may not be reproduced in any form without the express written permission of NCCN®. NCCN Guidelines Version 2.2013 Panel Members NCCN Guidelines Index MGF Table of Contents Myeloid Growth Factors Discussion * Jeffrey Crawford, MD/Chair †‡ Susan Hudock, PharmD å Lee S. Schwartzberg, MD † ‡ Þ Duke Cancer Institute The Sidney Kimmel Comprehensive St. Jude Children's Research Hospital/ Cancer Center at Johns Hopkins The University of Tennessee Health Science James Armitage, MD † x Center- The West Clinic UNMC Eppley Cancer Center at Dwight D. Kloth, PharmD å å The Nebraska Medical Center Fox Chase Cancer Center Sepideh Shayani, PharmD City of Hope Comprehensive Cancer Center Lodovico Balducci, MD † ‡ David J. Kuter, MD, DPhil † ‡ Moffitt Cancer Center Massachusetts General David P. Steensma, MD†‡Þ Hospital Cancer Center Dana-Farber/Brigham and Women’s Pamela Sue Becker, MD, PhD ‡ Þ x Cancer Center Fred Hutchinson Cancer Research Center/ Gary H. Lyman, MD, MPH † ‡ å Seattle Cancer Care Alliance Duke Cancer Institute Mahsa Talbott, PharmD Vanderbilt-Ingram Cancer Center Douglas W. Blayney, MD † Brandon McMahon, MD ‡ Stanford Cancer Institute Robert H. Lurie Comprehensive Cancer Saroj Vadhan-Raj, MD † Þ Center of Northwestern University The University of Texas Spero R. Cataland, MD ‡ MD Anderson Cancer Center The Ohio State University Comprehensive Hope S. Rugo, MD † ‡ x Cancer Center - James Cancer Hospital UCSF Helen Diller Family Peter Westervelt, MD, PhD † Siteman Cancer Center at Barnes- and Solove Research Institute Comprehensive Cancer Center Jewish Hospital and Washington University School of Medicine Mark L. -
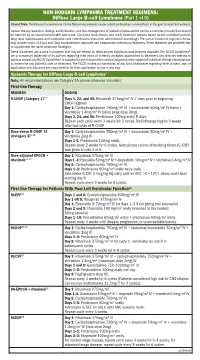
Diffuse Large B-Cell Lymphoma (Part 1 of 5)
NON-HODGKIN LYMPHOMA TREATMENT REGIMENS: Diffuse Large B-cell Lymphoma (Part 1 of 5) Clinical Trials: The National Comprehensive Cancer Network recommends cancer patient participation in clinical trials as the gold standard for treatment. Cancer therapy selection, dosing, administration, and the management of related adverse events can be a complex process that should be handled by an experienced health care team. Clinicians must choose and verify treatment options based on the individual patient; drug dose modifications and supportive care interventions should be administered accordingly. The cancer treatment regimens below may include both U.S. Food and Drug Administration-approved and unapproved indications/regimens. These regimens are provided only to supplement the latest treatment strategies. These Guidelines are a work in progress that may be refined as often as new significant data become available. The NCCN Guidelines® are a consensus statement of its authors regarding their views of currently accepted approaches to treatment. Any clinician seeking to apply or consult any NCCN Guidelines® is expected to use independent medical judgment in the context of individual clinical circumstances to determine any patient’s care or treatment. The NCCN makes no warranties of any kind whatsoever regarding their content, use, or application and disclaims any responsibility for their application or use in any way. Systemic Therapy for Diffuse Large B-cell Lymphoma1 Note: All recommendations are Category 2A unless otherwise indicated. First-line Therapy REGIMEN DOSING R-CHOP (Category 1)2–7 Days 1, 22, and 43: Rituximab 375mg/m2 IV 7 days prior to beginning CHOP regimen Day 1: Cyclophosphamide 750mg/m2 IV + doxorubicin 50mg/m2 IV bolus + vincristine 1.4mg/m2 IV bolus (max dose 2mg) Days 3, 24, and 45: Prednisone 100mg orally 5 days. -

Two Cases with CD-20 Negative Diffuse Large B-Cell Lymphoma and Literature Review
Open Access Annals of Hematology & Oncology Case Report Two Cases with CD-20 Negative Diffuse Large B-Cell Lymphoma and Literature Review Miranda-Aquino T*, Pérez-Topete SE and Montemayor-Montoya JL Abstract Department of Internal Medicine, Hospital Christus CD 20 negative diffuse large B-cell lymphoma is a very rare and aggressive Muguerza, Mexico neoplasm. We report two subtypes of this neoplasm: anaplastic lymphoma *Corresponding author: Tomas Miranda Aquino, kinase positive diffuse large B-cell lymphoma and a plasmablastic lymphoma. Department of Internal Medicine, Hospital Christus These pathologies are very difficult to diagnose and treat. The first case was Muguerza, UDEM, 1ra Av 758 Jardines de Anáhuac, San anaplastic lymphoma kinase positive diffuse large B-cell lymphoma, the patient Nicolás de los Garza, Nuevo León, México received multiple schemes of chemotherapy with a torpid evolution and the patient died. The second case is a plasmablastic lymphoma, the patient was Received: March 24, 2016; Accepted: May 18, 2016; treated with infusional etoposide, vincristine, doxorubicin, cyclophosphamide Published: May 20, 2016 and prednisone dose-adjusted chemotherapy, the treatment was successful with complete remission and without relapses. Keywords: CD 20 negative diffuses large B-cell lymphoma; Diffuse large B-cell lymphoma ALK positive; Plasmablastic lymphoma; Infusional DA- EPOCH; CHOP-Bleo Abbreviations and with a high proliferation index [2], with poor response to current therapies [3]. We report two cases with this diagnosis. -

(R*)-ICE ((Rituximab), Ifosfamide, Carboplatin and Etoposide) Therapy
NCCP Chemotherapy Regimen (R*)-ICE ((RiTUXimab), Ifosfamide, CARBOplatin and Etoposide) Therapy INDICATIONS FOR USE: Regimen Reimbursement INDICATION ICD10 Code Status Treatment of relapsed/refractory Non Hodgkin’s Lymphoma* C85 00397a Hospital Treatment of relapsed/refractory Hodgkin’s Lymphoma C81 00397b Hospital * RiTUXimab to be included in all CD20 positive patients TREATMENT: The starting dose of the drugs detailed below may be adjusted downward by the prescribing clinician, using their independent medical judgement, to consider each patients individual clinical circumstances. Treatment is administered on Day 1-3 as described in table every 21 days until remission induction or up to a maximum of 6 cycles. Facilities to treat anaphylaxis MUST be present when therapy is administered. Note: Specific Hydration therapy is required for the safe administration of aifosfamide (See Table below) Route and Method Cycle Day Drug Dose Diluent & Rate of Administration 1 RiTUXimab 375mg/m2 IV infusion1 500ml 0.9% NaCl at a maximum rate 1-6 Observe post of 400mg/hr1,3,4 infusion2 1, 2 , 3 Etoposide 100mg/m2 IV infusion 1000mls 0.9% NaCl over 60minutes 1-6 2 CARBOplatin AUC 5 IV infusion 500ml glucose 5% over 60 min 1-6 2 Mesna 1000mg/m2 IV Bolus Into the side arm of a fast-flowing 1-6 0.9% NaCl drip immediately before ifosfamide infusion starts 2 aIfosfamide 5000mg/ m2 IV infusion In 1000ml 0.9% NaCl over 24 hoursb 1-6 2 Mesna 5000mg/ m2 IV infusion In 1000ml 0.9% NaCl over 24 hours. 1-6 Y-sited with the ifosfamide 3 Mesna 1000mg/m2 IV bolus Into the side arm of a fast-flowing 1-6 0.9% NaCl drip 3 hours post end ifosfamide infusion 3 Mesna 1000mg/m2 IV bolus Into the side arm of a fast-flowing 1-6 0.9% NaCl drip 6 hours post end ifosfamide infusion 3 Mesna 1000mg/m2 IV bolus Into the side arm of a fast-flowing 1-6 0.9% NaCl drip 9 hours post end ifosfamide infusion From G-CSF 5mcg/kg SC Continued until ANC >1x109/L for 2 1-6 day 6 consecutive days (Round to nearest whole syringe) aIfosfamide Hydration: (Refer to local policy or see suggested hydration below). -

Anaplastic Large Cell Lymphoma in Children
Lymphomas in TYA Laurence Brugieres Département de cancérologie de l’enfant et de l’adolescent Gustave Roussy, Villejuif, France Lymphomas One of the most frequent tumors in TYA 120 Miscellaneous 100 Carcinoma 80 Germ cell tumor 60 Soft tissue sarcoma 40 Bone tumor Brain tumorTumeurs 20 cérébrales Lymphoma 0 15-19LNH 8%ans LNH20-24 16% ans Leucémies LH 20% LH 7% 15-19 years 20-24 yers 2000-2008 France, E.Desandes 2 Incidence of lymphomas according to age LNH Hodgkin Median age : 30 y 3.6% of the patients < 30 y Roman, Histopathology 2011 Lymphoma : therapeutic challenges ▪ 5-y survival rates over 90% in HL and 75 % in NHL in Europe (Trama 2016) ▪ Therapeutic challenges : – to early identify the small group of patients at high risk of failure requiring new therapeutic approaches – to reduce the burden of treatment in low and intermediate risk patients in order to limit short and long-term morbidity related to treatment. Lymphomas in TYA Heterogeneity of treatment according to age and site of care in patients in transition between childhood and adulthood 15-19 y 15-25 y 15-39 y Specific centers requirement ▪ a multi-professional approach ▪ opportunity to propose inclusion in trials available for this age group, ▪ fertility counselling, ▪ adapted psycho-social support ▪ a long-term follow-up in order to detect late morbidity of treatment staging Lymphomas TEP in initial staging Pediatric protocols Adult protocols ▪ optional for NHL ▪ Included in initial ▪ mandatory for HL staging for HL and NHL Ann Arbor classification I A single nodal or -

R-ICE NOTE: There Is a Modified RICE Protocol Which Must Be Used Within Oxford Due to Stability Restrictions with Baxter Aseptically Manufactured Ifosfamide
Lymphoma group R-ICE NOTE: there is a modified RICE protocol which must be used within Oxford due to stability restrictions with Baxter aseptically manufactured ifosfamide INDICATION Relapse treatment for relapsed or refractory lymphoma. Maybe used as part of frontline treatment (e.g. in secondary CNS lymphoma) Omit rituximab if CD20-negative. TREATMENT INTENT Curative. PRE-ASSESSMENT 1. Ensure histology is confirmed prior to administration of chemotherapy and document in notes. 2. Record stage of disease - CT scan (neck, chest, abdomen and pelvis), and/or PET-CT, presence or absence of B symptoms, clinical extent of disease, consider bone marrow aspirate and trephine. 3. Blood tests - FBC, ESR, DAT, U&Es, LDH, urate, calcium, magnesium, creatinine, LFTs, glucose, Igs, β2 microglobulin, hepatitis B core antibody and hepatitis B surface Ag, hepatitis C antibody, EBV, CMV, VZV, HIV 1+2 after consent. 4. See ‘Guidelines for the use of blood components in adult haematology’ for individual patient requirements. NB: All patients who are receiving priming chemotherapy for PBSC collection require irradiated blood products 7 days prior to harvest until harvest complete. Please send a “group and save” sample to blood transfusion and inform patient and transfusion laboratory +/- referring DGH need for requirements. Ensure irradiated blood product card is attached to patient’s notes and copy given to patient. 5. Urine pregnancy test • before cycle 1 of each new chemotherapy course for women of child bearing age unless they are postmenopausal, have been sterilised or undergone a hysterectomy. 6. ECG +/- Echo - if clinically indicated. 7. Record performance status (WHO/ECOG). 8. Record height and weight. -

Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients
Guideline for Classification of the Acute Emetogenic Potential of Antineoplastic Medication in Pediatric Cancer Patients POGO Antineoplastic – Induced Nausea and Vomiting Guideline Development Panel: L. Lee Dupuis MScPhm, ACPR, FCSHP Sabrina Boodhan BScPhm, ACPR Lillian Sung MD, PhD Carol Portwine MD, FRCPC, PhD Richard Hain MD Patricia McCarthy RN, (EC), MSc(A) Mark Holdsworth PharmD, BCOP The POGO Emetogenicity Classification Guidelines were developed by health care professionals using evidence-based or best practice references available at the time of their creation. Format and content of the guidelines will change as they are reviewed and revised on a periodic basis. Care has been taken to ensure accuracy of the information. However, every health care professional using these guidelines is responsible for providing care according to their best professional judgement and the policies and standards of care in place at their own institution. OVERVIEW OF MATERIAL Guideline Release Date: August 11, 2010 Status: Adapted, revised and updated Sources: Print copies available through POGO Electronic sources available through www.pogo.ca Adapters: POGO Antineoplastic-induced Nausea and Vomiting Guideline Development Panel POGO Emetogenicity Classification Guidelines TABLE OF CONTENTS Summary ............................................................................................................................................ 1 Glossary ............................................................................................................................................ -

Recommendations for Filgrastim Use in Adults by Disease Site
Practice Guideline: Clinical Guide Recommendations for Filgrastim Use in Adults by Disease Site Effective Date: June 2016 Updated: August 2018 CCMB Practice Guideline Clinical Guide Developed by: Breast, CNS, Gastrointestinal, Genitourinary, Gynecologic-Oncology, Hematology, Leukemia and Bone Marrow Transplant, Lymphoproliferative, Sarcoma and Thoracic Disease Site Groups CancerCare Manitoba Practice Guideline: Clinical Guide: Filgrastim| 2 Preface At CancerCare Manitoba (CCMB) the Clinical Practice Guidelines Initiative (CPGI) seeks to improve patient outcomes in terms of survival and quality of life through the development, dissemination, implementation, and evaluation of guidelines for the management of common clinical scenarios encountered by cancer patients throughout the province. This clinical guide was approved by the Breast, CNS, Gastrointestinal, Genitourinary, Gynecologic-Oncology, Hematology, Leukemia and Bone Marrow Transplant, Lymphoproliferative, Sarcoma and Thoracic Disease Site Groups. Purpose This document is intended as a guide to facilitate an evidence-informed, shared approach to the appropriate use of filgrastim in adults. For this purpose, it may be used by qualified and licensed healthcare practitioners involved with the care of oncology patients, which may include (but is not limited to): physicians, surgeons, nurses, radiation therapists, pharmacists, psychosocial oncology caregivers, and dieticians at CCMB, CCPN sites, Uniting Primary Care Oncology Network (UPCON) clinics, and WRHA Community Oncology Program sites. Disclaimer Use of this clinical guide in any setting should not preclude use of the practitioner’s independent clinical judgment; nor should it replace consultation with the appropriate oncology specialty when indicated (example: medical or radiation oncology, pharmacy, nursing, etc.). Clinicians are expected to apply the recommendations within boundaries of professional standards and scope of practice, and according to level of training and experience.