Irodalomjegyzék
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chlorophyta, Trebouxiophyceae) in Lake Tanganyika (Africa)*
Biologia 63/6: 799—805, 2008 Section Botany DOI: 10.2478/s11756-008-0101-4 Siderocelis irregularis (Chlorophyta, Trebouxiophyceae) in Lake Tanganyika (Africa)* Maya P. Stoyneva1, Elisabeth Ingolič2,WernerKofler3 &WimVyverman4 1Sofia University ‘St Kliment Ohridski’, Faculty of Biology, Department of Botany, 8 bld. Dragan Zankov, BG-1164 Sofia, Bulgaria; e-mail: [email protected], [email protected]fia.bg 2Graz University of Technology, Research Institute for Electron Microscopy, Steyrergasse 17,A-8010 Graz, Austria; e-mail: [email protected] 3University of Innsbruck, Institute of Botany, Sternwartestrasse 15,A-6020 Innsbruck, Austria; e-mail: werner.kofl[email protected] 4Ghent University, Department Biology, Laboratory of Protistology and Aquatic Ecology, Krijgslaan 281-S8,B-9000 Gent, Belgium; e-mail: [email protected] Abstract: Siderocelis irregularis Hindák, representing a genus Siderocelis (Naumann) Fott that is known from European temperate waters, was identified as a common phytoplankter in Lake Tanganyika. It was found aposymbiotic as well as ingested (possibly endosymbiotic) in lake heterotrophs, mainly Strombidium sp. and Vorticella spp. The morphology and ultrastructure of the species, studied with LM, SEM and TEM, are described with emphasis on the structure of the cell wall and the pyrenoid. Key words: Chlorophyta; cell wall; pyrenoid; symbiosis; ciliates; Strombidium; Vorticella Introduction ics of symbiotic species in general came into alignment with that of free-living algae and the term ‘zoochlorel- Tight partnerships between algae and aquatic inver- lae’ was abandoned as being taxonomically ambiguous tebrates, including symbiotic relationships, have long (e.g. Bal 1968; Reisser & Wiessner 1984; Taylor 1984; been of interest and a number of excellent reviews are Reisser 1992a). -

Globally Widespread Bryophytes, but Rare in Europe
Portugaliae Acta Biol. 20: 11-24. Lisboa, 2002 GLOBALLY WIDESPREAD BRYOPHYTES, BUT RARE IN EUROPE Tomas Hallingbäck Swedish Threatened Species Unit, P.O. Box 7007, SE-75007 Uppsala, Sweden. [email protected] Hallingbäck, T. (2002). Globally widespread bryophytes, but rare in Europe. Portugaliae Acta Biol. 20: 11-24. The need to save not only globally threatened species, but also regionally rare and declining species in Europe is discussed. One rationale of red-listing species regionally is to be preventive and to counteract the local species extinction process. There is also a value in conserving populations at the edge of their geographical range and this is discussed in terms of genetic variation. Another reason is the political willingness of acting locally rather than globally. Among the rare and non-endemic species in Europe, some are rare and threatened both in Europe and elsewhere, others are more common outside Europe and a third group is locally common within Europe but rare in the major part. How much conservation effort should be put on these three European non-endemic species groups is briefly discussed, as well as why bryophytes are threatened. A discussion is given, for example, of how a smaller total distribution range, decreasing density of localities, smaller sites, less substrate and lower habitat quality affect the survival of sensitive species. This is also compared with species that have either high or low dispersal capacity or different longevity of either vegetative parts or spores. Examples from Sweden are given. Key words: Bryophytes, rarity, Europe, dispersal capacity, Sweden. Hallingbäck, T. (2002). -

Neoproterozoic Origin and Multiple Transitions to Macroscopic Growth in Green Seaweeds
Neoproterozoic origin and multiple transitions to macroscopic growth in green seaweeds Andrea Del Cortonaa,b,c,d,1, Christopher J. Jacksone, François Bucchinib,c, Michiel Van Belb,c, Sofie D’hondta, f g h i,j,k e Pavel Skaloud , Charles F. Delwiche , Andrew H. Knoll , John A. Raven , Heroen Verbruggen , Klaas Vandepoeleb,c,d,1,2, Olivier De Clercka,1,2, and Frederik Leliaerta,l,1,2 aDepartment of Biology, Phycology Research Group, Ghent University, 9000 Ghent, Belgium; bDepartment of Plant Biotechnology and Bioinformatics, Ghent University, 9052 Zwijnaarde, Belgium; cVlaams Instituut voor Biotechnologie Center for Plant Systems Biology, 9052 Zwijnaarde, Belgium; dBioinformatics Institute Ghent, Ghent University, 9052 Zwijnaarde, Belgium; eSchool of Biosciences, University of Melbourne, Melbourne, VIC 3010, Australia; fDepartment of Botany, Faculty of Science, Charles University, CZ-12800 Prague 2, Czech Republic; gDepartment of Cell Biology and Molecular Genetics, University of Maryland, College Park, MD 20742; hDepartment of Organismic and Evolutionary Biology, Harvard University, Cambridge, MA 02138; iDivision of Plant Sciences, University of Dundee at the James Hutton Institute, Dundee DD2 5DA, United Kingdom; jSchool of Biological Sciences, University of Western Australia, WA 6009, Australia; kClimate Change Cluster, University of Technology, Ultimo, NSW 2006, Australia; and lMeise Botanic Garden, 1860 Meise, Belgium Edited by Pamela S. Soltis, University of Florida, Gainesville, FL, and approved December 13, 2019 (received for review June 11, 2019) The Neoproterozoic Era records the transition from a largely clear interpretation of how many times and when green seaweeds bacterial to a predominantly eukaryotic phototrophic world, creat- emerged from unicellular ancestors (8). ing the foundation for the complex benthic ecosystems that have There is general consensus that an early split in the evolution sustained Metazoa from the Ediacaran Period onward. -

Devonian Plant Fossils a Window Into the Past
EPPC 2018 Sponsors Academic Partners PROGRAM & ABSTRACTS ACKNOWLEDGMENTS Scientific Committee: Zhe-kun Zhou Angelica Feurdean Jenny McElwain, Chair Tao Su Walter Finsinger Fraser Mitchell Lutz Kunzmann Graciela Gil Romera Paddy Orr Lisa Boucher Lyudmila Shumilovskikh Geoffrey Clayton Elizabeth Wheeler Walter Finsinger Matthew Parkes Evelyn Kustatscher Eniko Magyari Colin Kelleher Niall W. Paterson Konstantinos Panagiotopoulos Benjamin Bomfleur Benjamin Dietre Convenors: Matthew Pound Fabienne Marret-Davies Marco Vecoli Ulrich Salzmann Havandanda Ombashi Charles Wellman Wolfram M. Kürschner Jiri Kvacek Reed Wicander Heather Pardoe Ruth Stockey Hartmut Jäger Christopher Cleal Dieter Uhl Ellen Stolle Jiri Kvacek Maria Barbacka José Bienvenido Diez Ferrer Borja Cascales-Miñana Hans Kerp Friðgeir Grímsson José B. Diez Patricia Ryberg Christa-Charlotte Hofmann Xin Wang Dimitrios Velitzelos Reinhard Zetter Charilaos Yiotis Peta Hayes Jean Nicolas Haas Joseph D. White Fraser Mitchell Benjamin Dietre Jennifer C. McElwain Jenny McElwain Marie-José Gaillard Paul Kenrick Furong Li Christine Strullu-Derrien Graphic and Website Design: Ralph Fyfe Chris Berry Peter Lang Irina Delusina Margaret E. Collinson Tiiu Koff Andrew C. Scott Linnean Society Award Selection Panel: Elena Severova Barry Lomax Wuu Kuang Soh Carla J. Harper Phillip Jardine Eamon haughey Michael Krings Daniela Festi Amanda Porter Gar Rothwell Keith Bennett Kamila Kwasniewska Cindy V. Looy William Fletcher Claire M. Belcher Alistair Seddon Conference Organization: Jonathan P. Wilson -

Ordovician Land Plants and Fungi from Douglas Dam, Tennessee
PROOF The Palaeobotanist 68(2019): 1–33 The Palaeobotanist 68(2019): xxx–xxx 0031–0174/2019 0031–0174/2019 Ordovician land plants and fungi from Douglas Dam, Tennessee GREGORY J. RETALLACK Department of Earth Sciences, University of Oregon, Eugene, OR 97403, USA. *Email: gregr@uoregon. edu (Received 09 September, 2019; revised version accepted 15 December, 2019) ABSTRACT The Palaeobotanist 68(1–2): Retallack GJ 2019. Ordovician land plants and fungi from Douglas Dam, Tennessee. The Palaeobotanist 68(1–2): xxx–xxx. 1–33. Ordovician land plants have long been suspected from indirect evidence of fossil spores, plant fragments, carbon isotopic studies, and paleosols, but now can be visualized from plant compressions in a Middle Ordovician (Darriwilian or 460 Ma) sinkhole at Douglas Dam, Tennessee, U. S. A. Five bryophyte clades and two fungal clades are represented: hornwort (Casterlorum crispum, new form genus and species), liverwort (Cestites mirabilis Caster & Brooks), balloonwort (Janegraya sibylla, new form genus and species), peat moss (Dollyphyton boucotii, new form genus and species), harsh moss (Edwardsiphyton ovatum, new form genus and species), endomycorrhiza (Palaeoglomus strotheri, new species) and lichen (Prototaxites honeggeri, new species). The Douglas Dam Lagerstätte is a benchmark assemblage of early plants and fungi on land. Ordovician plant diversity now supports the idea that life on land had increased terrestrial weathering to induce the Great Ordovician Biodiversification Event in the sea and latest Ordovician (Hirnantian) -

Answers to the Top 50 Questions About Genesis, Creation, and Noah's Flood
ANSWERS TO THE TOP 50 QUESTIONS ABOUT GENESIS, CREATION, AND NOAH’S FLOOD Daniel A. Biddle, Ph.D. Copyright © 2018 by Genesis Apologetics, Inc. E-mail: [email protected] www.genesisapologetics.com A 501(c)(3) ministry equipping youth pastors, parents, and students with Biblical answers for evolutionary teaching in public schools. The entire contents of this book (including videos) are available online: www.genesisapologetics.com/faqs Answers to the Top 50 Questions about Genesis, Creation, and Noah’s Flood by Daniel A. Biddle, Ph.D. Printed in the United States of America ISBN-13: 978-1727870305 ISBN-10: 1727870301 All rights reserved solely by the author. The author guarantees all contents are original and do not infringe upon the legal rights of any other person or work. No part of this book may be reproduced in any form without the permission of the author. The views expressed in this book are not necessarily those of the publisher. Scripture taken from the New King James Version®. Copyright © 1982 by Thomas Nelson. Used by permission. All rights reserved. Print Version November 2019 Dedication To my wife, Jenny, who supports me in this work. To my children Makaela, Alyssa, Matthew, and Amanda, and to your children and your children’s children for a hundred generations—this book is for all of you. We would like to acknowledge Answers in Genesis (www.answersingenesis.org), the Institute for Creation Research (www.icr.org), and Creation Ministries International (www.creation.com). Much of the content herein has been drawn from (and is meant to be in alignment with) these Biblical Creation ministries. -

JUDD W.S. Et. Al. (2002) Plant Systematics: a Phylogenetic Approach. Chapter 7. an Overview of Green
UNCORRECTED PAGE PROOFS An Overview of Green Plant Phylogeny he word plant is commonly used to refer to any auto- trophic eukaryotic organism capable of converting light energy into chemical energy via the process of photosynthe- sis. More specifically, these organisms produce carbohydrates from carbon dioxide and water in the presence of chlorophyll inside of organelles called chloroplasts. Sometimes the term plant is extended to include autotrophic prokaryotic forms, especially the (eu)bacterial lineage known as the cyanobacteria (or blue- green algae). Many traditional botany textbooks even include the fungi, which differ dramatically in being heterotrophic eukaryotic organisms that enzymatically break down living or dead organic material and then absorb the simpler products. Fungi appear to be more closely related to animals, another lineage of heterotrophs characterized by eating other organisms and digesting them inter- nally. In this chapter we first briefly discuss the origin and evolution of several separately evolved plant lineages, both to acquaint you with these important branches of the tree of life and to help put the green plant lineage in broad phylogenetic perspective. We then focus attention on the evolution of green plants, emphasizing sev- eral critical transitions. Specifically, we concentrate on the origins of land plants (embryophytes), of vascular plants (tracheophytes), of 1 UNCORRECTED PAGE PROOFS 2 CHAPTER SEVEN seed plants (spermatophytes), and of flowering plants dons.” In some cases it is possible to abandon such (angiosperms). names entirely, but in others it is tempting to retain Although knowledge of fossil plants is critical to a them, either as common names for certain forms of orga- deep understanding of each of these shifts and some key nization (e.g., the “bryophytic” life cycle), or to refer to a fossils are mentioned, much of our discussion focuses on clade (e.g., applying “gymnosperms” to a hypothesized extant groups. -

The Green Puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New Generic and Species Concept Among This Widely Distributed Genus
Phytotaxa 441 (2): 113–142 ISSN 1179-3155 (print edition) https://www.mapress.com/j/pt/ PHYTOTAXA Copyright © 2020 Magnolia Press Article ISSN 1179-3163 (online edition) https://doi.org/10.11646/phytotaxa.441.2.2 The green puzzle Stichococcus (Trebouxiophyceae, Chlorophyta): New generic and species concept among this widely distributed genus THOMAS PRÖSCHOLD1,3* & TATYANA DARIENKO2,4 1 University of Innsbruck, Research Department for Limnology, A-5310 Mondsee, Austria 2 University of Göttingen, Albrecht-von-Haller-Institute of Plant Sciences, Experimental Phycology and Sammlung für Algenkulturen, D-37073 Göttingen, Germany 3 [email protected]; http://orcid.org/0000-0002-7858-0434 4 [email protected]; http://orcid.org/0000-0002-1957-0076 *Correspondence author Abstract Phylogenetic analyses have revealed that the traditional order Prasiolales, which contains filamentous and pseudoparenchy- matous genera Prasiola and Rosenvingiella with complex life cycle, also contains taxa of more simple morphology such as coccoids like Pseudochlorella and Edaphochlorella or rod-like organisms like Stichococcus and Pseudostichococcus (called Prasiola clade of the Trebouxiophyceae). Recent studies have shown a high biodiversity among these organisms and questioned the traditional generic and species concept. We studied 34 strains assigned as Stichococcus, Pseudostichococcus, Diplosphaera and Desmocococcus. Phylogenetic analyses using a multigene approach revealed that these strains belong to eight independent lineages within the Prasiola clade of the Trebouxiophyceae. For testing if these lineages represent genera, we studied the secondary structures of SSU and ITS rDNA sequences to find genetic synapomorphies. The secondary struc- ture of the V9 region of SSU is diagnostic to support the proposal for separation of eight genera. -
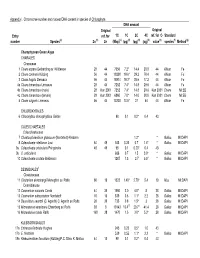
Green Appendix I
Appendix I. Chromsome number and nuclear DNA content in species of Chlorophyta DNA amount Original Original Entry ref. for 1C 1C 2C 4C ref. for C- Standard number Species(a) 2n (b) 2n (Mbp)(c) (pg)(d) (pg)(d) (pg)(d) value(e) species(f) Method(g) Charophycean Green Algae CHARALES Characeae 1 Chara aspera Detharding ex Willdenow 28 44 7056 7.2* 14.4 28.8 44 Allium Fe 2 Chara contraria Kützing 56 44 19208 19.6* 39.2 78.4 44 Allium Fe 3 Chara fragilis Desvaux 56 44 18914 19.3* 38.6 77.2 44 Allium Fe 4a Chara tomentosa Linnaeus 28 44 7252 7.4* 14.8 29.6 44 Allium Fe 4b Chara tomentosa (male) 28 Kun 2001 7252 7.4* 14.8 29.6 Kun 2001 Chara MI:EB 4c Chara tomentsoa (female) 28 Kun 2001 6860 7.0* 14.0 28.0 Kun 2001 Chara MI:EB 5 Chara vulgaris Linnaeus 56 44 13230 13.5* 27 54 44 Allium Fe CHLOROKYBALES 6 Chlorokybus atmosphyticus Geitler 98 0.1 0.2* 0.4 43 COLEOCHAETALES Coleochaetaceae 7 Chaetosphaeridium globosum (Nordstedt) Klebahn 1.2* * Gallus MI:DAPI 8 Coleochaete nitellarum Jost 84 49 343 0.35 0.7 1.4* * Gallus MI:DAPI 9a Coleochaete orbicularis Pringsheim 48 49 98 0.1 0.20* 0.4 43 9b C. orbicularis 686 0.7 1.5 3.0* * Gallus MI:DAPI 10 Coleochaete scutata Brébisson 1287 1.3 2.7 5.5* * Gallus MI:DAPI DESMIDIALES1 Closteriaceae 11 Closterium ehrenbergii Meneghini ex Ralfs 60 19 1323 1.40* 2.70* 5.4 19 Mus MI:DAPI Desmidiaceae 12 Cosmarium cucumis Corda 44 39 1960 2.0 4.0* 8 28 Gallus MI:DAPI 13 Cosmarium subcostatum Nordstedt 10 16 539 0.6 1.1* 2.2 28 Gallus MI:DAPI 14 Desmidium swartzii (C. -

A Symbiosis with Fungi?
BIO Web of Conferences 4, 00009 (2015) DOI: 10.1051/bioconf/20150400009 C Owned by the authors, published by EDP Sciences, 2015 Origins of the terrestrial flora: A symbiosis with fungi? Marc-André Selosse1,a and Christine Strullu-Derrien2 1 Institut de Systématique, Évolution, Biodiversité (ISYEB - UMR 7205 – CNRS, MNHN, UPMC, EPHE), Muséum national d’Histoire naturelle, Sorbonne Universités, 57 rue Cuvier, CP. 50, 75005 Paris, France 2 Department of Earth Sciences, The Natural History Museum, Cromwell Road, London SW7 5BD, UK Abstract. Land phototrophs need to exploit both atmosphere (providing gas and light) and substrate (furnishing water and minerals). Yet, their algal ancestors were poorly pre- adapted to such a life at the interface. We review the paleontological evidence that fungal symbioses which can exploit substrate resources, helped adaptation to land constraints. Diverse structures dating back to the Devonian present convincing evidence for lichens, (symbioses between fungi and microscopic algae) but fossils remain scarce, so that early lichen abundance and ecological relevance remain questionable. Several enigmatic but abundant fossils from the Siluro-Devonian, such as Spongiophyton or the giant Prototaxites (Nematophytes), likely represent fungus-algal symbioses, which shaped early terrestrial ecosystems. Yet, these taxa are fully extinct, and do not have clear affinities with extant groups. Finally, terrestrialization of Embryophyta (land plants), which currently dominate land ecosystems, is linked to a symbiosis with Glomeromycetes. Today, these fungi form arbuscular mycorrhizae, which help most Embryophyta to exploit soil, and molecular data combined with paleontological evidence support the idea that this type of association is ancestral. The role of symbiotic Mucoromycetes during terrestrialization is not fully understood and mycorrhizal association diversified later in the evolution of Embryophyta. -

Iop Newsletter 120
IOP NEWSLETTER 120 October 2019 CONTENTS Letter from the president Elections of IOP Executive Committee 2020 – Call for nominations Special issue in occasion of the 65th birthday of Hans Kerp Collection Spotlight: Cleveland Museum of Natural History Reflections from the ‘Earth Day’ 2019 Upcoming meetings IOP Logo: The evolution of plant architecture (© by A. R. Hemsley) 1 Letter from the president Greetings Members, This past quarter we have welcomed the publication Festschrifts celebrating our colleagues Hans Kerp (PalZ Paläontologische Zeitschrift, see below) and Gar Rothwell (International Journal of Plant Sciences v 180 nos. 7, 8). Thank you to the teams of editors and authors who have contributed to these issues illustrating the continuing strength of our discipline— and congratulations to Hans and Gar for inspiring such productivity by your admiring colleagues! Already three years have passed since our gathering for IOPC in Salvador, Brazil in 2016, and now planning is in full swing for next year’s meeting in Prague, 12-19 September 2020. This will be the 11th quadrennial meeting of IOP, held concurrently with the 15th International Palynological Conference. Please note the formal announcement in this newsletter (page 12, herein). Nominations for colleagues deserving of honorary membership are welcome at any time. As the submission of abstracts for IOPC/IPC presentations will be possible soon, we kindly remind the IOP Student Travel Awards. We will financially support about 5 to 7 PhD or MSc students in order to enable them to participate in the conference and present their research results in a talk. Recent PhD graduates will also qualify for these awards, if their completion was less than nine months prior to the time of the conference. -

Nomen Est Omen — Die Bedeutung Der Art- Und Unterart-Epitheta Der Schweizer Moosflora
Zurich Open Repository and Archive University of Zurich Main Library Strickhofstrasse 39 CH-8057 Zurich www.zora.uzh.ch Year: 2014 Nomen est omen: die Bedeutung der Art- und Unterart-Epitheta der Schweizer Moosflora Urmi, Edi Posted at the Zurich Open Repository and Archive, University of Zurich ZORA URL: https://doi.org/10.5167/uzh-103870 Journal Article Published Version Originally published at: Urmi, Edi (2014). Nomen est omen: die Bedeutung der Art- und Unterart-Epitheta der Schweizer Moosflora. Meylania, (53):3-80. Nomen est omen — Die Bedeutung der Art- und Unterart-Epitheta der Schweizer Moosflora von Edi Urmi Meylania 53 (2014) Editorial Nomen est omen — Die Bedeutung der Art- und Unter- art-Epitheta der Schweizer Moosflora Dass die Beschäftigung mit der Herkunft der botanischen Namen eine spannende Sache ist, hat uns schon Josef Bertram 2005 mit der Sondernummer 32/33 der Meylania gezeigt: „Herkunft und Bedeutung der Gattungsnamen der in Deutschland, E. Urmi in der Schweiz und in Österreich vorkommenden Moose“. Dass die Faszination Meylania 53 (2014): 3-80 ungebrochen ist, beweist uns nun Edwin Urmi in dieser Sondermeylania, die als Ergänzung zu den Gattungsnamen die Bedeutung der Artnamen zum Inhalt hat. Wer wissen möchte, was der wissenschaftliche Name Anthoceros punctatus bedeu- Der Autor hat akribisch unsere ganze Moosflora durchgekämmt. Artnamen für tet, sucht zunächst Aufklärung in der Sondernummer 32/33 der Meylania. Dort Artnamen hat er analysiert und versucht, die Bedeutung der jeweiligen Namen zu hat Josef Bertram in vorbildlicher Weise z. B. den Gattungsnamen Anthoceros er- deuten. Häufig war die Bedeutung recht naheliegend, manchmal ging der Weg aber klärt (BERTRAM 2005).