Meningitis and Meningoencephalitis (Pdf)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unusual Case of Progressive Multifocal Leukoencephalopathy in a Patient with Sjögren Syndrome
Henry Ford Health System Henry Ford Health System Scholarly Commons Pathology Articles Pathology 1-15-2021 Unusual Case of Progressive Multifocal Leukoencephalopathy in a Patient With Sjögren Syndrome Ifeoma Onwubiko Kanika Taneja Nilesh S. Gupta Abir Mukherjee Follow this and additional works at: https://scholarlycommons.henryford.com/pathology_articles CASE REPORT Unusual Case of Progressive Multifocal Leukoencephalopathy in a Patient With Sjögren Syndrome Ifeoma Ndidi Onwubiko, MD, MPH, Kanika Taneja, MD, Nilesh Gupta, MD, and Abir Mukherjee, MD 03/05/2021 on BhDMf5ePHKav1zEoum1tQfN4a+kJLhEZgbsIHo4XMi0hCywCX1AWnYQp/IlQrHD3i3D0OdRyi7TvSFl4Cf3VC1y0abggQZXdgGj2MwlZLeI= by http://journals.lww.com/amjforensicmedicine from Downloaded 84% neutrophils; glucose, 71 mmol/L; protein, 183 g/dL with neg- Abstract: Progressive multifocal leukoencephalopathy (PML) is a rare Downloaded ative cultures and no malignant cells on cytology). Hepatitis C anti- demyelinating disease caused by reactivation of John Cunningham virus af- body screen was negative. Immunoglobin G antibodies to Sjögren fecting typically subcortical and periventricular white matter of immunocom- syndrome–related antigen A and anti–Sjögren syndrome-related from promised hosts (human immunodeficiency virus infection, hematologic antigen B were positive. Thyroglobulin antibodies and antinuclear http://journals.lww.com/amjforensicmedicine malignancies). Cerebral hemispheric white matter is most commonly affected antibodies were elevated. Autoimmune serological tests for other by lytic -

CONTENTS EDITORIAL Chronic Fatigue Syndrome
Volume 11 Number 1 2003 CONTENTS EDITORIAL Chronic Fatigue Syndrome Guidelines 1 Kenny De Meirleir Neil McGregor Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols 7 Bruce M. Carruthers Anil Kumar Jain Kenny L. De Meirleir Daniel L. Peterson Nancy G. Klimas A. Martin Lerner Alison C. Bested Pierre Flor-Henry Pradip Joshi A. C. Peter Powles Jeffrey A. Sherkey Marjorie I. van de Sande Recent years have brought growing recognition of the need for clinical criteria for myalgic encephalomyelitis (ME), which is also called chronic fatigue syndrome (CFS). An Expert Subcommittee of Health Canada established the Terms of Refer- ence, and selected an Expert Medical Consensus Panel representing treating physi- cians, teaching faculty and researchers. A Consensus Workshop was held on March 30 to April 1, 2001 to culminate the review process and establish consen- sus for a clinical working case definition, diagnostic protocols and treatment pro- tocols. We present a systematic clinical working case definition that encourages a diagnosis based on characteristic patterns of symptom clusters, which reflect specific areas of pathogenesis. Diagnostic and treatment protocols, and a short overview of research are given to facilitate a comprehensive and integrated ap- proach to this illness. Throughout this paper, “myalgic encephalomyelitis” and “chronic fatigue syndrome” are used interchangeably and this illness is referred to as “ME/CFS.” KEYWORDS. Clinical case definition, myalgic encephalomyelitis, chronic fa- tigue syndrome, ME, CFS, diagnostic protocol, treatment protocol Monitoring a Hypothetical Channelopathy in Chronic Fatigue Syndrome: Preliminary Observations 117 Jo Nijs Christian Demanet Neil R. McGregor Pascale De Becker Michel Verhas Patrick Englebienne Kenny De Meirleir This study was aimed at monitoring of a previously suggested channelopathy in Chronic Fatigue Syndrome, and at searching for possible explanations by means of immune system characteristics. -

Progressive Multifocal Leukoencephalopathy and the Spectrum of JC Virus-Related Disease
REVIEWS Progressive multifocal leukoencephalopathy and the spectrum of JC virus- related disease Irene Cortese 1 ✉ , Daniel S. Reich 2 and Avindra Nath3 Abstract | Progressive multifocal leukoencephalopathy (PML) is a devastating CNS infection caused by JC virus (JCV), a polyomavirus that commonly establishes persistent, asymptomatic infection in the general population. Emerging evidence that PML can be ameliorated with novel immunotherapeutic approaches calls for reassessment of PML pathophysiology and clinical course. PML results from JCV reactivation in the setting of impaired cellular immunity, and no antiviral therapies are available, so survival depends on reversal of the underlying immunosuppression. Antiretroviral therapies greatly reduce the risk of HIV-related PML, but many modern treatments for cancers, organ transplantation and chronic inflammatory disease cause immunosuppression that can be difficult to reverse. These treatments — most notably natalizumab for multiple sclerosis — have led to a surge of iatrogenic PML. The spectrum of presentations of JCV- related disease has evolved over time and may challenge current diagnostic criteria. Immunotherapeutic interventions, such as use of checkpoint inhibitors and adoptive T cell transfer, have shown promise but caution is needed in the management of immune reconstitution inflammatory syndrome, an exuberant immune response that can contribute to morbidity and death. Many people who survive PML are left with neurological sequelae and some with persistent, low-level viral replication in the CNS. As the number of people who survive PML increases, this lack of viral clearance could create challenges in the subsequent management of some underlying diseases. Progressive multifocal leukoencephalopathy (PML) is for multiple sclerosis. Taken together, HIV, lymphopro- a rare, debilitating and often fatal disease of the CNS liferative disease and multiple sclerosis account for the caused by JC virus (JCV). -

Relapsing Polychondritis Presenting with Meningoencephalitis and Dementia: Correlation with Neuroimaging and Clinical Features
30 Relapsing Polychondritis Presenting with Meningoencephalitis and Dementia: Correlation with Neuroimaging and Clinical Features Yung-Pin Hwang1, Richard Kuo2,4, Tien-Ling Chen3, Pei-Hao Chen1,4,5, Shih-Jung Cheng1 Abstract- Purpose: Relapsing polychondritis (RP) is a rare systemic autoimmune disease affecting cartilaginous and non-cartilaginous structures. Neurological involvement is rarer but results in profound disability. Early identification and treatment of underlying RP may promote neurological recovery. Case Report: We illustrated a 53-year-old man diagnosed with dementia. Neuroimaging and cerebrospinal fluid studies disclosed meningoencephalitis. “Prominent ear sign” was evident on diffusion-weight magnetic resonance imaging. After glucocortisone administration, the improvement of clinical manifestations was closely correlated subsequent neuroimaging findings. Conclusion: The importance of better understanding of this disease in terms of the prevention of further tissue damage in patients with RP cannot be overemphasized. Key Words: relapsing polychondritis, auricular chondritis, meningoncephalitis, dementia, prominent ear sign Acta Neurol Taiwan 2015;24:30-33 INTRODUCTION of RP are protean dependent on tissue involvement. Early identification of PR and prompt intervention might be Relapsing polychondritis (RP) is a rare multisystem beneficial for its prognosis. Nervous system involvement disorder affecting cartilaginous tissue including the hyaline can manifest as meningoencephalitis (4), dementia (5), stroke cartilage of joints and the fibrocartilage of extra-articular (6), and parkinsonism (7). Awareness of RP may prevent location, as well as proteoglycan-rich structures such the subsequent disability secondary to irreversible brain or (1) media of the arteritis and eye . Its pathogenesis appears nerve tissue damage. to be an immune-mediated reaction against collagen type We report a case with meningoencephalitis and (2) (3) II , collagens type IX and XI . -
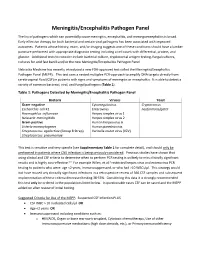
Meningitis/Encephalitis Pathogen Panel
Meningitis/Encephalitis Pathogen Panel The list of pathogens which can potentially cause meningitis, encephalitis, and meningoencephalitis is broad. Early effective therapy for both bacterial and certain viral pathogens has been associated with improved outcomes. Patients whose history, exam, and/or imaging suggests one of these conditions should have a lumber puncture performed with appropriate diagnostic testing including a cell count with differential, protein, and glucose. Additional tests to consider include bacterial culture, cryptococcal antigen testing, fungal cultures, cultures for acid fast bacilli and/or the new Meningitis/Encephalitis Pathogen Panel. Nebraska Medicine has recently introduced a new FDA-approved test called the Meningitis/Encephalitis Pathogen Panel (MEPP). This test uses a nested multiplex PCR-approach to amplify DNA targets directly from cerebrospinal fluid (CSF) in patients with signs and symptoms of meningitis or encephalitis. It is able to detect a variety of common bacterial, viral, and fungal pathogens (Table 1). Table 1: Pathogens Detected by Meningitis/Encephalitis Pathogen Panel Bacteria Viruses Yeast Gram-negative Cytomegalovirus Cryptococcus Escherichia coli K1 Enterovirus neoformans/gattii Haemophilus influenzae Herpes simplex virus 1 Neisseria meningitidis Herpes simplex virus 2 Gram-positive Human herpesvirus 6 Listeria monocytogenes Human parechovirus Streptococcus agalactiae (Group B Strep) Varicella zoster virus (VZV) Streptococcus pneumoniae This test is sensitive and very specific (see Supplementary Table 1 for complete detail), and should only be performed in patients where CNS infection is being seriously considered. Previous studies have shown that using clinical and CSF criteria to determine when to perform PCR testing is unlikely to miss clinically significant results and is highly cost-effective.1-3 For example Wilen, et al.3 restricted herpes virus and enterovirus PCR testing to patients who were: age <2 years, immunosuppressed, or who had >10 WBCs/µl. -

Inflammation of the Central Nervous System- Encephalitis, Myelitis, and Meningitis
Inflammation of the Central Nervous System- Encephalitis, Myelitis, and Meningitis Stacy Dillard, DVM, Diplomate ACVIM (Neurology) Inflammation of the central nervous system is a very common condition seen in veterinary neurology. The area in which the inflammation predominates indicates which of the clinical syndromes we use to name the disease. Inflammation involving the brain is called encephalitis, of the spinal cord is called myelitis and of the meninges is called meningitis. It is common to have more than one area of the central nervous system affected and therefore combinations of the names are often used such as meningoencephalomyelitis or meningoencephalitis. Encephalitis is the most common area of the central nervous system to be affected and will be used as the general term in this article. Etiology There are two main classes of encephalitis: infectious and idiopathic. Infectious etiologies include viruses, fungi, bacteria, protozoa, tick borne diseases and even algae. True infectious encephalitis is much less common in the dog than in other species including humans; however diagnosis is critical for proper targeted therapy. Idiopathic encephalitis means that an infectious etiology has not been found and encompasses a wide range of syndromes appreciated in the canine patient. Many idiopathic encephalitidies are thought to be immune-mediated and respond well to immune-suppression. Other idiopathic encephalitis, such as necrotizing encephalitis found in young toy breed dogs, do not appear to respond as well to immuno- suppression and therefore may represent another form of the disease. Idiopathic encephalitis is much more common in the dog than infectious encephalitis; however, definitive diagnosis is critical as treatment is markedly different between the two categories of disease. -

Zika Virus Meningoencephalitis and Myelitis and Associated Magnetic Resonance Imaging Findings
Am. J. Trop. Med. Hyg., 97(2), 2017, pp. 340–343 doi:10.4269/ajtmh.16-0921 Copyright © 2017 by The American Society of Tropical Medicine and Hygiene Case Report: Zika Virus Meningoencephalitis and Myelitis and Associated Magnetic Resonance Imaging Findings Faruq Pradhan,1* Joseph D. Burns,2 Ahmed Agameya,1 Avignat Patel,3 Mohammad Alfaqih,4 Juan E. Small,4 and Winnie Ooi5 1Department of Internal Medicine, Lahey Hospital and Medical Center, Burlington, Massachusetts; 2Department of Neurology, Lahey Hospital and Medical Center, Burlington, Massachusetts; 3Department of Pulmonary and Critical Care Medicine, Lahey Hospital and Medical Center, Burlington, Massachusetts; 4Department of Radiology, Lahey Hospital and Medical Center, Burlington, Massachusetts; 5Department of Infectious Diseases, Lahey Hospital and Medical Centre, Travel and Tropical Medicine Clinic, Burlington, Massachusetts Abstract. Zika virus (ZIKV) has a wide clinical spectrum of associated neurologic disease including microcephaly and Guillain–Barre syndrome but, despite its known neurotropism, ZIKV meningoencephalitis and myelitis have been rare complications. We describe a case of ZIKV meningoencephalitis and probable myelitis and its associated magnetic resonance imaging findings that rapidly resolved during recovery in a previously healthy adult. INTRODUCTION stretch reflexes were 3+ diffusely with clonus at the ankles, and plantar reflexes were extensor bilaterally. Pregnancy The geographical distribution of Zika virus (ZIKV) has testing was negative. Doxycycline was added to her regimen. fi steadily broadened since the virus was rst detected in Ampicillin and dexamethasone were discontinued. Uganda in 1947 and now includes parts of the United States A repeat lumbar puncture was performed on day 2. CSF fl where Aedes aegypti exists. -

Case Reports Acute Disseminated Encephalomyelitis Following Streptococcus Pneumoniae Meningoencephalitis
HK J Paediatr (new series) 2013;18:37-41 Case Reports Acute Disseminated Encephalomyelitis Following Streptococcus Pneumoniae Meningoencephalitis YL YU, HF LI, ZZ XIA, F GAO Abstract Acute disseminated encephalomyelitis (ADEM) is an autoimmune demyelinating disease of the central nervous system. We report a 4-year-old girl who suffered from acute meningoencephalitis caused by Streptococcus pneumoniae (S. pneumoniae). After 8 days of treatment by sensitive antibiotics, the cerebrospinal fluid (CSF) cell count, blood count and the serum level of C-reactive protein recovered quickly, while the level of CSF protein was still high, with continuous coma and fever. Magnetic resonance imaging indicated multifocal changes in the brain parenchyma, mainly in white matter, with bithalamic involvement. The CSF IgG index increased, oligoclonal bands were positive. After high-dose intravenous methylprednisolone and intravenous immunoglobulin were given, the patient came around, with normal CSF results and body temperature. Within a few months the patient recovered completely and there were no relapses during nearly 3 years of follow-up. To our knowledge, this may be the first report of ADEM following S. pneumoniae meningoencephalitis in children. Key words Acute disseminated encephalomyelitis (ADEM); Streptococcus pneumoniae Introduction meningoencephalitis reported.4-6 S. pneumoniae is the main pathogens of bacterial pneumonia and meningitis in Acute disseminated encephalomyelitis (ADEM) is an children. To our knowledge, there was no report of ADEM autoimmune disease characterised by an inflammatory following S. pneumoniae infection confirmed in children. reaction and demyelination in the central nervous system We report on a patient diagnosed as ADEM following (CNS) that usually develops following acute viral S. -

Clinical Presentation of Meningitis in Adults
Clinical Presentation of Meningitis in Adults Prof. Dr. Serhat Ünal FACP, FEFIM Hacettepe University, Faculty of Medicine Department of© Infectious by author Diseases , ANKARA Meningitis Update ESCMIDESCMID PostgraduateOnline Lecture Educational Library Course September 2013, İzmir Why Is Clinical Examination Important? "If, in a fever, the neck be turned awry on a sudden, so that the sick can hardly swallow, and yet no tumour appear, it is mortal.- © by author ESCMID“Aphorism Online XXXV Lecture of Hippocrates Library” Meningitis • Meningitis is a clinical syndrome characterized by inflammation of the meninges • Infectious Meningitis – caused by a variety of infectious agents • bacteria, viruses, fungi, and parasites. • Clinical signs and symptoms at presentation may predict prognosis • Only 25% of adults© by have author a classic presentation and are not a diagnostic dilemma. • ESCMIDMany patients Online have a Lecture less obvious Library presentation Mace SE, Emerg Med Clin N Am 2008;38:281 Spanos A et al JAMA 1998;262:2700 Clinicians Suspecting Meningitis • While taking the patient's history • Examine for – General symptoms of infection • such as fever, chills, and myalgias – Symptoms suggesting central nervous system infection © by author • photophobia, headache, nausea and vomiting, focal neurologic symptoms, or changes in mental status ESCMID Online Lecture Library Clinical Presantation of Meningitis (Dept. Of Emergency) The suspicion of ABM is critically dependent on the early recognition of the meningitis syndrome. • 156 patients with meningitis -Taiwan – I nitial ED diagnosis was correct in only 58% of the cases. • The 3 most common© by alternative author diagnoses – Nonmeningeal infection ESCMID– Metabolic encephalopathy Online Lecture Library – Nonspecific conditions Chern CH, Ann Emerg Med. -

Mumps Meningoencephai'tis a Clinical Review of 119 Cases with One Death HENRY B
Mumps Meningoencephai'tis A Clinical Review of 119 Cases with One Death HENRY B. BRUYN, M.D., HAROLD M. SEXTON. M.D., and HENRY D. BRAINERD, M.D., San Francisco MUMPS IS A generalized, systemic, virus infection in * Mumps is one of the most common viruses to which the salivary glands are commonly affected and affect the central nervous system and should be given primary consideration in the differential in which inflammation of the central nervous system diagnosis of aseptic meningitis. Many cases of occurs with such frequency that it can hardly be mumps infection do not involve the salivary considered a complication. In fact, the virus of glands. mumps could be said to be one of the most common The course of mumps meningoencephalitis is viral agents to affect the central nervous system. De- usually benign, with fever and signs of menin- geal irritation lasting less than five days. The spite the extensive literature attesting these facts, findings in the cerebrospinal fluid are usually there still exists the widespread impression that distinctive, with leukocyte content greater than mumps meningoencephalitis is a rare complication. 200 per milliliter, of which 80 per cent or more Hamilton10 in 1790 presented one of the first for- are lymphocytes. Sequelae, even of a minor na- ture, are rare. mal reports of "A Distemper, By the Common Peo- Death is extremely rare in recorded literature. ple in England Vulgarly Called the Mumps." In this A fatal case of mumps meningoencephalitis is very carefully written report he described the usual described herein. course of this disease and stressed the frequency of central nervous system involvement. -

JCV) Seroprevalence Among Zambian Adults Presenting with “Meningoencephalitis” to the University Teaching Hospital, Lusaka, Zambia
Medical Journal of Zambia, Vol. 46 (4): 277 - 285 (2019) Original Article A Study of the John Cunningham Virus (JCV) Seroprevalence among Zambian Adults presenting with “Meningoencephalitis” to the University Teaching Hospital, Lusaka, Zambia 1A. Patel, 2S. Lakhi, 1, 3 O. Siddiqi, 4I. Koralnik 1 University of Zambia, School of medicine, Department of Internal Medicine, Lusaka, Zambia 2 University Teaching Hospital, Department of Internal Medicine, Lusaka, Zambia 3 Department of Neurology, Beth Israel Deaconess Medical Centre, Boston, USA 4 Department of Neurological Sciences, Rush University Medical Centre, Chicago, USA ABSTRACT Results: Final analysis for JCV seroprevalence was done in 96 patients and noted to be 46% (95% CI, Background: The John Cunningham virus (JCV) is 35.62 – 56.31). The JCV seroprevalence in the HIV an opportunistic virus which leads to the positive group was 40.82% and in the HIV negative development of progressive multifocal group was 51.06 % but there was no statistical leukoencephalopathy (PML), which is a lytic difference (p-value 0.31). None of the other factors infection of oligodendrocytes of the central nervous studied had any impact on the JCV seroprevalence system in immunosuppressed patients. Infection such as age, gender, clinical presentation, CD4 with the JCV occurs in childhood and the virus count and co-morbid TB meningitis (TBM) remains quiescent in the body, activating during diagnosis. There was a bimodal distribution of age immunosuppression. Exposure to the virus can be associated with JCV seropositivity; with one peak detected by testing for JC virus specific antibodies in occurring in the 18 to 20 years age group and the an ELISA test. -

Acute Inflammatory Myelopathies
UCSF UC San Francisco Previously Published Works Title Acute inflammatory myelopathies. Permalink https://escholarship.org/uc/item/3wk5v9h9 Journal Handbook of clinical neurology, 122 ISSN 0072-9752 Author Cree, Bruce AC Publication Date 2014 DOI 10.1016/b978-0-444-52001-2.00027-3 Peer reviewed eScholarship.org Powered by the California Digital Library University of California Handbook of Clinical Neurology, Vol. 122 (3rd series) Multiple Sclerosis and Related Disorders D.S. Goodin, Editor Copyright © 2014 Bruce Cree. Published by Elsevier B.V. All rights reserved Chapter 28 Acute inflammatory myelopathies BRUCE A.C. CREE* Department of Neurology, University of California, San Francisco, USA INTRODUCTION injury caused by the acute inflammation and the likeli- hood of recurrence differs depending on the etiology. Spinal cord inflammation can present with symptoms sim- Additional important diagnostic and prognostic features ilar to those of compressive myelopathies: bilateral weak- include whether the myelitis is partial or transverse, ness and sensory changes below the spinal cord level of febrile illness, the number of vertebral spinal cord injury, often accompanied by bowel and bladder impair- segments involved on MRI at the time of acute attack, ment and sparing cranial nerve and cerebral function. the rapidity from symptom onset to maximum deficit, Because of the widespread availability of magnetic reso- and the severity of involvement. nance imaging (MRI) and computed tomography (CT) imaging, compressive etiologies can be rapidly excluded, METHODOLOGIC CONSIDERATIONS leading to the consideration of non-compressive etiologies for myelopathy. The differential diagnosis of non- Large observational cohort studies or randomized con- compressive myelopathy is broad and includes infectious, trolled trials concerning myelitis have never been under- parainfectious, toxic, nutritional, vascular, and systemic taken.