From Microbubbles to Nanobubbles: Effect on Flotation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Making Decisions About Water and Wastewater for Aqueous Operation
Making Decisions about Water and Wastewater for Aqueous Operation John F. Russo Chapter 2.17 Handbook for Critical Cleaning Editor-in-Chief Barbara Kanegsberg Reprinted with permission from CRC Press www.crcpress.com INTRODUCTION..................................................................................................................................3 TYPICAL CLEANING SYSTEM............................................................................................................3 OPERATIONAL SITUATIONS OF TYPICAL USER ...............................................................................4 Determining the Water Purity Requirements .........................................................................................4 Undissolved Contaminants............................................................................................................4 Dissolved Contaminants...............................................................................................................4 Undissolved and Dissolved Contaminants........................................................................................5 Other Conditions...........................................................................................................................5 Determining the Wastewater Volume Produced .....................................................................................6 Source Water Trea tment .....................................................................................................................6 No -

Refinery Wastewater Management Using Multiple Angle Oil-Water Separators
REFINERY WASTEWATER MANAGEMENT USING MULTIPLE ANGLE OIL-WATER SEPARATORS Kirby S. Mohr, P.E. Mohr Separations Research, Inc. 1278 FM 407 Suite 109 Lewisville, TX 75077 Phone: 918-299-9290 Cell: 918-269-8710 John N. Veenstra, Ph.D., P.E., Oklahoma State University Dee Ann Sanders, Ph.D., P.E. Oklahoma State University A paper presented at the International Petroleum Environment Conference in Albuquerque, New Mexico, 1998 ABSTRACT In this work, an overview of oil-water separation, as used in the petroleum refining industries, is presented along with case studies. Discussions include: impact of solids, legal aspects, and differing types of systems currently in use, along with their advantages and disadvantages. Performance information on separators is presented with an emphasis on new multiple angle coalescing plate technology for refinery wastewater management. Several studies are presented including a large (20,000 US GPM flow rate) system recently installed at a major US refinery. The separator was constructed by converting two existing API separators into four separators, and adding multiple angle coalescing plates to increase throughput and efficiency. A year of operating experience with this system indicates good performance and few problems. Other examples provide information on separators installed in the United States and other countries. Keywords: Oil-water separator, multiple angle, coalescence, refinery, wastewater management, petroleum, coalescing plate technology BACKGROUND AND INTRODUCTION Oil has been refined for various uses for at least 1000 years. An Arab handbook written by Al-Razi, in approximately 865 A.D., describes distillation of “naft” (naphtha) for use in lamps and thus the beginning of oil refining (Forbes). -

New Technologies for Water and Wastewater Treatment: a Survey of Recent Patents Berrin Tansel*
Recent Patents on Chemical Engineering, 2008, 1, 17-26 17 New Technologies for Water and Wastewater Treatment: A Survey of Recent Patents Berrin Tansel* Florida International University, Civil and Environmental Engineering Department, Engineering Center 3600, Miami, Florida 33174, USA Received: July 31, 2007; Accepted: September 19, 2007; Revised: November 12, 2007 Abstract: The concern over increasing needs for drinking water and awareness for development of systems to improve water quality both for drinking purposes and for effluents from wastewater treatment and industrial facilities have provided incentives to develop new technologies and improve performance of existing technologies. In this paper, the patents on treatment of water and wastewater approved during the period from 1999 to 2007 were reviewed. The patents surveyed were classified into two groups as technologies for water purification systems for drinking water, and technologies for treatment of wastewater. An assessment of the current and future outlook for development of new technologies, methods of treatment, equipment and instruments which can be used for water and wastewater treatment applications are presented. Keywords: Water treatment, water filtration, ultrapure water, wastewater treatment, ion exchange, disinfection, sorption, membrane filtration, nanofiltration, wastewater. 1. INTRODUCTION 2. WATER TREATMENT SYSTEMS FOR DRINKING Water is an essential substance for living systems as it WATER allows the transport of nutrients and waste products in living The general treatment of drinking water takes place in systems. Research shows a clear correlation between several steps to remove dissolved and suspended solids. The diseases and the amount and types of fluids consumed, treatment processes may include processes such as floccu- health-promoting properties of nutrients which can be added to water, optimal intake levels, and consumption patterns. -

Liquid / Solids Separation in Wastewater Treatment & Biosolids Dewatering
LIQUID / SOLIDS SEPARATION IN WASTEWATER TREATMENT & BIOSOLIDS DEWATERING Chemical Products Lab Testing Plant Trials LIQUID / SOLIDS SEPARATION APPLICATIONS Influent Water Clarification Process Water Recycling Primary Wastewater Clarification Secondary Clarification Sludge Thickening Sludge Dewatering LIQUID / SOLIDS SEPARATION UNIT OPERATIONS Clarifiers (Many Types) WATER Filters (Many Types) OR WASTE Dissolved Air Flotation Units WATER Induced Air/Gas Flotation Units Belt Presses Centrifuges SLUDGE Screw Presses DEWATERING Plate and Frame Presses Vacuum Filters (Rotary & Horizontal) LIQUID / SOLIDS SEPARATION PRODUCT TYPES Coagulants (+) Low Mol Wt Organic Inorganic Blended Flocculants (+ , ---, 0 ) High Mol Wt Dry Emulsion Solution OilOil----FreeFree Flocculants COAGULANTS AND FLOCCULANTS Act on Insoluble Particles in Water Oils, Grease, Blood, Insoluble Organics, Clay, Silicates, Metal Oxides/Hydroxides Dirt, Dust, Rust & Metal Filings Can Act on Charged Organic Compounds Anionic Surfactants, Soaps & Dispersants Do Not Act on Most Dissolved Solids Salts, Acids, Nonionic Surfactants, Ammonia or Soluble Organic Compounds such as Sugar, Alcohols, etc. SUSPENSION CHEMISTRY THE KEY TO EFFECTIVE LIQUID / SOLIDS SEPARATION SUSPENDED SOLIDS VARIABLES Surface Charge MOST Charge Density Particle Size IMPORTANCE Composition Particle Density Particle Shape LEAST MICROSCOPIC FORCES ELECTROSTATIC BROWNIAN VAN DER WAALS GRAVITY Colloidal Particle in Water +++ +++ +++ +++ +++ +++ +++ +++ +++ +++ Almost all Particles +++ -

Simulation of the Open Sky Seawater Distillation
Green and Sustainable Chemistry, 2013, 3, 68-88 http://dx.doi.org/10.4236/gsc.2013.32012 Published Online May 2013 (http://www.scirp.org/journal/gsc) The Best Available Technology of Water/Wastewater Treatment and Seawater Desalination: Simulation of the Open Sky Seawater Distillation Djamel Ghernaout Chemical Engineering Department, Saad Dahlab University of Blida, Blida, Algeria Email: [email protected] Received March 16, 2013; revised April 18, 2013; accepted April 26, 2013 Copyright © 2013 Djamel Ghernaout. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT This review suggests the concept of the best available technology of water/wastewater treatment and seawater desalina- tion which is in fact a simulation of the seawater distillation at the open sky: coagulation in salty water aerated basin/ coagulation using seawater as coagulant solution with distillation using stored solar energy followed by waterfall on a natural mountain. This natural, green, and technico-economical technology is composed of three steps: the first one is coagulation which may be achieved: 1) in salty water aerated basin (air stripping, AS; dissolved air flotation, DAF) where the raw water is “diluted” in seawater; or 2) in “conventional” coagulation using seawater as coagulant solution instead of alum/ferric salts. The first option seems to be more natural as it simulates river water dilution in seawater and the second one is more practical for “rapid” water consummation. For colloids and microorganisms’ removal, double- layer compression and charge neutralisation, as main coagulation and disinfection mechanisms, would be involved in the first and second options, respectively. -

Dissolved Air Flotation
PPU Umwelttechnik GMBH PPU Umwelttechnik GMBH is a technology company based in Bavaria, Germany. We develop unique wastewater treatment processes, including physical, biological, chemical, filtration and oxidation steps. We offer products, and complete tailor made solutions for any industrial/municipal application. We manufacture all of our products and solutions at our factory in Bavaria. ClearFox® ClearFox® is the brand name for the range of products and solutions manufactured by PPU. ClearFox® products are exported all over the world. Systems are currently installed and operating in Africa, Europe, Middle East, Russia/CIS and Asia. The ClearFox® brand is globally recognised as a specialist provider of solutions for wastewater treatment Contact Us Carl-Kolb-Str. 6 95448 Bayreuth, Germany DAF +49 (0) 921150 63 99 0 [email protected] Dissolved air www.clearfox.com flotation Advanced physical and chemical industrial wastewater treatment The next generation of DAF, bringing an established process technology to the next level, with higher efficiencies and lower costs Reference Projects A unique, highly efficient dissolved air flotation process smaller footprint, high efficiency and lower chemical usage than conventional DAF systems ClearFox Advanced DAF A compact and efficient solution for all industrial wastewaters from 1-500m3/hr Highly efficient pollutant removal for a range of industries and sectors including: - Food and beverage, - Pharmaceutical UK - Food and Beverage Egypt - Oil and Gas Germany - Landfill - Oil and gas, - PET, A modular DAF installed to reduce Specialist DAF modules for the A high rate DAF used in conjunction - Wood, COD, BOD and TSS from a fish cleaning of produced water from with other ClearFox solutions for - Landfill, factory in the UK with 5m3/hr oil drilling sites across Egypt. -

Dissolved Air Flotation Systems
PALMETTO WATER SOLUTIONS DISSOLVED AIR FLOTATION SYSTEMS A DISSOLVED AIR FLOTATION SYSTEM (DAF) DESIGNED TO MEET ANY SOLIDS REMOVAL REQUIREMENTS Palmetto Water Solutions’ DAF systems are designed to The Palmetto Water Solutions DAFs are offered with remove suspended solids, oil, and grease, heavy metals, varying levels of automation ranging from manual relay BOD, COD and VOCs from a wide variety of industrial logic control to fully automated PLC controlled systems with waste streams. They achieve this by utilizing a unique air SCADA monitoring and data logging capabilities. dissolving system to produce a high level of air saturation and extremely small micro bubbles in the process water. Features include: Microbubbles are entrained by flocculating in the presence • Mild steel, stainless steel or polypropylene construction of dissolved air. This causes them to rapidly float to the • Skid mounted, compact footprint surface of the DAF for removal by a sludge raking system, • Proven air dissolving technology requires no pressure leaving only the clarified water behind. vessel • Advanced flocculator design provides fast polymer Palmetto Water Solutions designs and manufactures DAF dosage diagnostics systems ranging from small pilot systems to high flow • Low wear sludge scraping components industrial wastewater treatment systems with flow rates • Internal sludge hopper ranging from 10 to 1,000 gpm (0.04 to 3.8 m3/min). Palmetto Water Solutions manufactures DAF treatment systems with the following optional features: • PLC automation • SCADA -

Hydrodynamic Aspects of Flotation Separation
Open Chem., 2016; 14: 132–139 Review Article Open Access Efrosyni N. Peleka* and Kostas A. Matis Hydrodynamic aspects of flotation separation DOI 10.1515/chem-2016-0014 received January 8, 2016; accepted April 6, 2016. ii) Dissolved-air flotation (DAF), clarifies wastewaters by removal of suspended oil or solids. Abstract: Flotation separation is mainly used for removing Air is dissolved in the wastewater under pressure and particulates from aqueous dispersions. It is widely used for released in a flotation tank. The resulting tiny bubbles ore beneficiation and recovering valuable materials. This adhere to the suspended matter and float to the surface paper reviews the hydrodynamics of flotation separations where it is removed by skimming. DAF is widely used in and comments on selected recent publications. Units are treating effluents from refineries, petrochemical and distinguished as cells of ideal and non-ideal flow. A brief chemical plants, natural gas processing plants, paper mills, introduction to hydrodynamics is included to explain an similar industrial facilities, and general water treatment. original study of the hybrid flotation-microfiltration cell, Flotation is a very complicated process combining effective for heavy metal ion removal. fundamental hydrodynamics with many elementary physicochemical steps (bubble-particle interaction forces, Keywords: fine particles, flotation, flow pattern, particle-particle interaction forces etc.). Its modelling is hydrodynamics, metal ions removal, water, wastewater, important to understand -
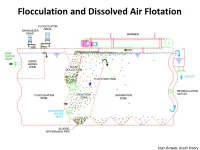
Flocculation and Dissolved Air Flotation
Flocculation and Dissolved Air Flotation Sean Bowen, Aleah Henry Cost Estimation Biomass production/yr 25 tonnes Tank Surface Area 940.5 m2 Yearly operation period 330 days (18h/d) Energy costs $7692 per year Flocculant $15000/year Capital interest for investment 16.5%/yr Unit Description Includes: tankage, pumps, motors, internal piping, c/s package unit, building, air compressor, mix tank, control panel Total BM Cost 2011 (assume error $4,722,400 +-20%) [1] US Department of Energy. National Algal Biofuels Technology Roadmap. Energy Efficiency and Renewable Energy. May 2010. Pages 37-101 [2] Chun-Yen Chen, Kuei-Ling Yeh, Rifka Aisyah, Duu-Jong Lee, Jo-Shu Chang, Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review, Bioresource Technology, Volume 102, Issue 1, January 2011, Pages 71-81 Rotary Vacuum Drum Washers In Kraft Mill Pulping • Each washer operates at 80% filtering efficiency therefore 3 washer in series are connected • In separation of pulp it is the cake formation • Approximately 7 tons of black liquor form for each ton of pulp Design Criteria • Minimum cake thickness is 6 mm • Effective filtration rate per unit area of the drum surface area depends on Figure 2: Rotary vacuum drum submerged circumference filter • The drum takes up 푚 푝푢푙푝 approximately 15% by 퐴푑푟푢푚 = 푚 푓푖푙푡,푓푖푛 volume of the entire washer unit • Rotation speed depends on the time spent in each zone Vacuum Drum Washer Costs Equipment Costs Wash water costs Ultrafiltration • based on the ability of pressure-driven filtration membranes to separate multicomponent solutes according to molecular size, shape, and chemical bonding • Hydraulic pressure drives substances with a smaller molecular size through a membrane while the larger molecules are held back Annual Operating Costs • Capital Cost for Ultrafiltration Membrane Separation unit: $31,324 to $73,088 one time fee. -

Petroleum Refining Water/Wastewater Management
Petroleum refining Operations Best Practice water/wastewater Series use and management 2010 www.ipieca.org The global oil and gas industry association for environmental and social issues 5th Floor, 209–215 Blackfriars Road, London SE1 8NL, United Kingdom Telephone: +44 (0)20 7633 2388 Facsimile: +44 (0)20 7633 2389 E-mail: [email protected] Internet: www.ipieca.org © IPIECA 2010 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without the prior consent of IPIECA. This publication is printed on paper manufactured from fibre obtained from sustainably grown softwood forests and bleached without any damage to the environment. Petroleum refining water/wastewater use and management IPIECA Operations Best Practice Series This document was prepared by AECOM, Inc. on behalf of the IPIECA Refinery Water Management Task Force. The assistance of M. Venkatesh of ENSR–AECOM is gratefully acknowledged. Cover photographs reproduced courtesy of the following (clockwise from top left): ExxonMobil; Nexen; Photodisc Inc.; iStockphoto; Corbis; Shutterstock.com. IPIECA Contents Introduction 2 Effluent treatment 25 Process wastewater pretreatment 25 Refinery water overview 3 Desalter effluent treatment 25 Overall refinery water balance 3 Wastewater segregation 27 Sources of water 3 Primary treatment 28 Water leaving the refinery 4 First stage: separation Raw water treatment: 5 (oil/water separators, API separators) -

Technologies & Services for the Treatment of Water, Liquor And
Technologies & Services for the Treatment of Water, Liquor and Chemical Recovery Cycles for the Pulp & Paper Industry WATER TECHNOLOGIES Understanding the Water is vital to the production process at Pulp Capabilities Applications and Paper facilities. Veolia Water Technologies Veolia is experienced in the design Challenges Facing understands the issues related to water and and implementation of standard and > Absorption Pulp & Paper wastewater management and has experience customized technologies and processes for > Aerobic Treatment in helping manufacturers achieve their goals in: the Pulp and Paper industry > Anaerobic Treatment Companies > Biogas Cleaning/Handling > Chemicals > Regulatory Compliance Black Liquor Concentration > Clarification > Operational Efficiency The thermal treatment of black liquor through evaporation achieves > Coagulation / Flocculation higher solids content and lower loads to the recovery boiler leading > Increased Resource Recovery to the optimization of combined heat and power production. When > Crystallization > Risk Mitigation integrated within the chemical recovery cycle, this technology > Dealkalization promotes higher operational efficiencies while enabling the reuse > Corporate Sustainability Initiatives > Demineralization of high-quality condensate. > Dissolved Air Flotation > Equalization Precipitator Ash Treatment > Evaporation With the closing of process loops, chloride and potassium > Ion Exchange A Creative and Trusted Veolia Water Technologies is trusted by leading accumulate in the recovery cycle -

Effect of Coagulation and Sonication on the Dissolved Air Flotation (DAF) Process for Thickening of Biological Sludge in Wastewater Treatment
Environmental Health Engineering and Management Journal 2020, 7(1), 59–65 doi 10.34172/EHEM.2020.08 http://ehemj.com Environmental Health H E M J Engineering and Management Journal Original Article Open Access Publish Free Effect of coagulation and sonication on the dissolved air flotation (DAF) process for thickening of biological sludge in wastewater treatment Ali Atamaleki1 ID , Mohammad Bagher Miranzadeh1 ID , Gholam Reza Mostafaii1* ID , Hosein Akbari2 ID , Leila Iranshahi1 ID , Farshid Ghanbari3 ID , Atieh Salem1 ID 1Department of Environmental Health Engineering, School of Public Health, Kashan University of Medical Sciences, Kashan, Iran 2Biostatistics and Public Health Department, Faculty of Health, Kashan University of Medical Sciences, Kashan, Iran 3Abadan Faculty of Medical Sciences, Abadan, Iran Abstract Article History: Background: Dissolved air floatation (DAF) is one of the methods has been used for the sludge Received: 15 November 2019 thickening in wastewater treatment plants. This study aimed to investigate the effects of coagulation Accepted: 12 February 2020 and sonication processes as additional configurations on the efficiency of a lab-scale DAF process for ePublished: 29 February 2020 thickening of the biological sludge of an industrial wastewater treatment plant in Kashan, Iran. Methods: The required amounts of sludge samples were collected from a wastewater treatment plant and kept at temperature of 4°C. Variables, such as pressure (3, 5, and 7 atm), flotation time (5 and 10 minutes), ultrasonic irradiation power (0, 75, and 150 W), and presence/absence of Fe-based coagulant were considered on a sequencing batch reactor (SBR) included coagulation, flotation, and sonication processes, respectively. Results: The use of ultrasonic waves led to an insignificant increase in the DAF efficiency (P > 0.05), however, the application of coagulant significantly increased the thickening efficiency (P < 0.05).