Sizing Ocean Giants: Patterns of Intraspecific Size Variation in Marine Megafauna
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
Accelerated Reader Quiz List
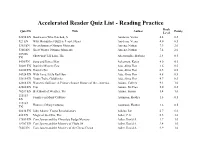
Accelerated Reader Quiz List - Reading Practice Book Quiz ID Title Author Points Level 32294 EN Bookworm Who Hatched, A Aardema, Verna 4.4 0.5 923 EN Why Mosquitoes Buzz in People's Ears Aardema, Verna 4.0 0.5 5365 EN Great Summer Olympic Moments Aaseng, Nathan 7.9 2.0 5366 EN Great Winter Olympic Moments Aaseng, Nathan 7.4 2.0 107286 Show-and-Tell Lion, The Abercrombie, Barbara 2.4 0.5 EN 5490 EN Song and Dance Man Ackerman, Karen 4.0 0.5 50081 EN Daniel's Mystery Egg Ada, Alma Flor 1.6 0.5 64100 EN Daniel's Pet Ada, Alma Flor 0.5 0.5 54924 EN With Love, Little Red Hen Ada, Alma Flor 4.8 0.5 35610 EN Yours Truly, Goldilocks Ada, Alma Flor 4.7 0.5 62668 EN Women's Suffrage: A Primary Source History of the...America Adams, Colleen 9.1 1.0 42680 EN Tipi Adams, McCrea 5.0 0.5 70287 EN Best Book of Weather, The Adams, Simon 5.4 1.0 115183 Families in Many Cultures Adamson, Heather 1.6 0.5 EN 115184 Homes in Many Cultures Adamson, Heather 1.6 0.5 EN 60434 EN John Adams: Young Revolutionary Adkins, Jan 6.7 6.0 480 EN Magic of the Glits, The Adler, C.S. 5.5 3.0 17659 EN Cam Jansen and the Chocolate Fudge Mystery Adler, David A. 3.7 1.0 18707 EN Cam Jansen and the Mystery of Flight 54 Adler, David A. 3.4 1.0 7605 EN Cam Jansen and the Mystery of the Circus Clown Adler, David A. -

Winter 2016 in This Issue Photo: Alex Constan Alex Photo: from the President in These Cold Winter Months, I Often Pause to Enjoy the Aquarium’S Tropical Exhibits
It’s time to live blue™ The Phoenix Islands Protected Area Meet Myrtle, the queen of the Giant Ocean Tank New England’s undersea treasure Members’ Magazine Volume 49, Number 1 Winter 2016 In This Issue Photo: Alex Constan Alex Photo: From the President In these cold winter months, I often pause to enjoy the Aquarium’s tropical exhibits. The colors and abundance of life consis- tently delight me and also remind me of how vulnerable these systems are. In this issue of blue, we’ll journey to one of the most remote tropical coral reef systems on the planet. In September, a team of scientists from the Aquarium, the Woods Hole Oceano- graphic Institution and other collaborators visited the Phoenix Islands Protected Area (PIPA), one of the largest marine protected areas in the world. They collected data that will help to manage the reserve and— in the midst of one of the most intense El Niños ever—observed the effects of climate change, without the complicating Cool Animals Future Ocean Protectors factors that affect most coral reef systems. 2 6 Myrtle the green sea turtle Nature is so weird. Did you know? Closer to home, our conservation team has been raising awareness of two extraordinary gems off our own coasts: 3 Research Briefs 8 Global Explorers Cashes Ledge, an underwater mountain A potential pregnancy test for Researchers return to the range that supports New England’s long-dead whales, and the growing Phoenix Islands Protected Area largest and deepest kelp forest, and the problem of big fish in home tanks Coral Canyons and Seamounts, home to 10 Members’ Notes rare deep sea corals and invertebrates. -

Thermoregulation Strategies of Deep Diving Ectothermic Sharks
THERMOREGULATION STRATEGIES OF DEEP DIVING ECTOTHERMIC SHARKS A DISSERTATION SUBMITTED TO THE GRADUATE DIVISION OF THE UNIVERSITY OF HAWAIʻI AT MĀNOA IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCOTOR OF PHILOSOPHY IN ZOOLOGY (MARINE BIOLOGY) AUGUST 2020 By. Mark A. Royer Dissertation Committee: Kim Holland, Chairperson Brian Bowen Carl Meyer Andre Seale Masato Yoshizawa Keywords: Ectothermic, Thermoregulation, Biologging, Hexanchus griseus, Syphrna lewini, Shark ACKNOWLEDGEMENTS Thank you to my advisor Dr. Kim Holland and to Dr. Carl Meyer for providing me the privilege to pursue a doctoral degree in your lab, which provided more experiences and opportunities than I could have ever imagined. The research environment you provided allowed me to pursue new frontiers in the field and take on challenging questions. Thank you to my committee members Dr. Brian Bowen, Dr. Andre Seale, and Dr. Masato Yoshizawa, for providing your ideas, thoughts, suggestions, support and encouragement through the development of my dissertation. I would like to give my sincere thanks to all of my committee members and to the Department of Biology for taking their time to provide their support and accommodation as I finished my degree during a rather unprecedented and uncertain time. I am very grateful to everyone at the HIMB Shark Lab including Dr. Melanie Hutchinson, Dr. James Anderson, Jeff Muir, and Dr. Daniel Coffey. I learned so much from all of you and we have shared several lifetimes worth of experiences. Thank you to Dr. James Anderson for exciting side projects we have attempted and will continue to pursue in the future. Thank you to Dr. -

Winter 2014 on the Cover: Northern Fur Seals Photo: K
It’s time to live blue™ A female fur seal is born at the Aquarium Aquarium scientists search for new ways to protect right whales Going solar Members’ Magazine Volume 47, Number 1 Winter 2014 On the cover: Northern fur seals Photo: K. Ellenbogen blue is a quarterly magazine exclusively for members of the New England Aquarium produced and published by New England Aquarium, Central Wharf, Boston, MA, 02110. Publishing office Aquarium researchers are experimenting with ways to keep right whales like this one from getting entangled in fishing located at 177 Milk St., line. In this magazine all photographs of right whales in U.S. waters were taken under NMFS/NOAA permit under the Boston, MA, 02109. blue authority of the Marine Mammal Protection Act and the U.S. Endangered Species Act. and all materials within are property of the New England Aquarium. Reproduction of any In This Issue @neaq.org materials is possible only through written Dive into a sea of resources online. www.neaq.org permission. Cool Animal: Kitovi 2 Meet our newest Northern fur seal. The website is full of conservation information, © blue 2014 animal facts and details that will help you plan your next trip to the Aquarium. Editor live blue™: Solar Panels and Ann Cortissoz 4 Citizen Scientists Throughout this issue of blue, look for Designer Cathy LeBlanc this icon to point out items that you Future Ocean Protectors: can explore further on our website. Contributors 6 Emily Bauernfeind Using Colors to Save Right Whales Jeff Ives Scientists search for new methods to help Plan Your Visit Scott Kraus these endangered giants. -

Migration and Habitat Utilization in Lamnid Sharks
MIGRATION AND HABITAT UTILIZATION IN LAMNID SHARKS A DISSERTATION SUBMITTED TO THE DEPARTMENT OF BIOLOGICAL SCIENCES AND THE COMMITTEE ON GRADUATE STUDIES OF STANFORD UNIVERSITY IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY Kevin Chi-Ming Weng May 2007 © Copyright by Kevin Chi-Ming Weng 2007 All Rights Reserved ii Abstract Understanding the movements, habitat utilization, and life history of high trophic level animals is essential to understanding how ecosystems function. Furthermore, large pelagic vertebrates, including sharks, are declining globally, yet the movements and habitats of most species are unknown. A variety of satellite telemetry techniques are used to elucidate the movements and habitat utilization of two species of lamnid shark. Salmon sharks used a subarctic to subtropical niche, and undertook long distance seasonal migrations between subarctic and subtropical regions of the eastern North Pacific, exhibiting the greatest focal area behavior in the rich neritic waters off Alaska and California, and showing more transitory behaviors in pelagic waters where productivity is lower. The timing of salmon shark aggregations in both Alaska and California waters appears to correspond with life history events of an important group of prey species, Pacific salmon. The enhanced expression of excitation-contraction coupling proteins in salmon shark hearts likely underlies its ability to maintain heart function at cold temperatures and their niche expansion into subarctic seas. Adult white sharks undertake long distance seasonal migrations from the coast of California to an offshore focal area 2500 km west of the Baja Peninsula, as well as Hawaii. A full migration cycle from the coast to the offshore focal area and back was documented. -

Thermoregulation in the Leatherback Sea Turtle (Dermochelys Coriacea)
Thermoregulation in the leatherback sea turtle (Dermochelys coriacea ) by Brian Lee Bostrom B.Sc., The University of British Columbia, 2005 A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES (Zoology) THE UNIVERSITY OF BRITISH COLUMBIA (Vancouver) August 2009 © Brian Lee Bostrom, 2009 ABSTRACT Adult leatherback turtles ( Dermochelys coriacea ) exhibit thermal gradients between their bodies and the environment of ≥ 8 °C in sub-polar waters and ≤ 4 °C in the tropics. There has been no direct evidence for thermoregulation in leatherbacks although modelling and morphological studies have given an indication of how thermoregulation may be achieved. Using a cylindrical model of a leatherback I investigated the extent to which heat production by muscle activity during variation of swim speed could be used in a leatherback’s thermal strategy. Drag force of a full scale cast of a leatherback was measured in a low velocity wind tunnel to obtain an estimate of the metabolic cost needed to offset drag. It is apparent, from this modelling, that heat flux from the body and flippers, activity and body and water temperatures are important variables to measure in order to fully classify the thermoregulatory response of live leatherbacks. Using captive juvenile leatherbacks of 16 and 37 kg I show for the first time that leatherbacks are indeed capable of thermoregulation. In cold water (< 25 °C), flipper stroke frequency increased, heat loss through the plastron, carapace and flippers was minimized, and a positive thermal gradient of up to 2.3 °C was maintained between body and environment. -

Implications for Foraging Efficiency and Thermoregulation
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 15 September 2015 doi: 10.3389/fmars.2015.00064 Swimming strategy and body plan of the world’s largest fish: implications for foraging efficiency and thermoregulation Mark G. Meekan 1*, Lee A. Fuiman 2, Randall Davis 3, Yuval Berger 1 and Michele Thums 1 1 The Australian Institute of Marine Science, Crawley, WA, Australia, 2 Marine Science Institute, The University of Texas at Austin, Port Aransas, TX, USA, 3 Department of Marine Biology, Texas A&M University, Galveston, TX, USA The largest animals in the oceans eat prey that are orders of magnitude smaller than themselves, implying strong selection for cost-effective foraging to meet their energy demands. Whale sharks (Rhincodon typus) may be especially challenged by warm seas Edited by: Graeme Clive Hays, that elevate their metabolism and contain sparse prey resources. Using a combination Deakin University, Australia of biologging and satellite tagging, we show that whale sharks use four strategies Reviewed by: to save energy and improve foraging efficiency: (1) fixed, low power swimming, (2) Gail Schofield, Deakin University, Australia constant low speed swimming, (3) gliding, and (4) asymmetrical diving. These strategies Nuno Queiroz, increase foraging efficiency by 22–32% relative to swimming horizontally and resolve University of Porto, Portugal the energy-budget paradox of whale sharks. However, sharks in the open ocean must Michael Brian Bennett, ◦ The University of Queensland, access food resources that reside in relatively cold waters (up to 20 C cooler than the Australia surface) at depths of 250–500 m during the daytime, where long, slow gliding descents, *Correspondence: continuous ram ventilation of the gills and filter-feeding could rapidly cool the circulating Mark G. -

Isotopic Ordering in Eggshells Reflects Body Temperatures And
ARTICLE Received 19 Oct 2014 | Accepted 7 Aug 2014 | Published 13 Oct 2015 DOI: 10.1038/ncomms9296 Isotopic ordering in eggshells reflects body temperatures and suggests differing thermophysiology in two Cretaceous dinosaurs Robert A. Eagle1,2,3,4, Marcus Enriquez1, Gerald Grellet-Tinner5,6, Alberto Pe´rez-Huerta7, David Hu2, Thomas Tu¨tken8, Shaena Montanari9, Sean J. Loyd1,10, Pedro Ramirez11, Aradhna K. Tripati1,3,4,12, Matthew J. Kohn13, Thure E. Cerling14, Luis M. Chiappe15 & John M. Eiler2 Our understanding of the evolutionary transitions leading to the modern endothermic state of birds and mammals is incomplete, partly because tools available to study the thermo- physiology of extinct vertebrates are limited. Here we show that clumped isotope analysis of eggshells can be used to determine body temperatures of females during periods of ovulation. Late Cretaceous titanosaurid eggshells yield temperatures similar to large modern endo- therms. In contrast, oviraptorid eggshells yield temperatures lower than most modern endotherms but B6 °C higher than co-occurring abiogenic carbonates, implying that this taxon did not have thermoregulation comparable to modern birds, but was able to elevate its body temperature above environmental temperatures. Therefore, we observe no strong evidence for end-member ectothermy or endothermy in the species examined. Body tem- peratures for these two species indicate that variable thermoregulation likely existed among the non-avian dinosaurs and that not all dinosaurs had body temperatures in the range of that seen in modern birds. 1 Department of Earth, Planetary, and Space Sciences, University of California, Los Angeles, California 90095, USA. 2 Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California 91125, USA. -

EDUCATOR GUIDE EDUCATOR GUIDE Humpback Fun Facts
Macgillivray Freeman’s presented by pacific life EDUCATOR GUIDE EDUCATOR GUIDE Humpback fun Facts WEIGHT At birth: 1 ton LENGTH Adult: 25 - 50 tons Up to 55 feet, with females larger DIET than males; newborns are Krill, about 15 feet long small fish APPEARANCE LIFESPAN Gray or black, with white markings 50 to 90 years on their undersides THREATS Entanglement in fishing gear, ship strikes, habitat impacts 4 EDUCATOR GUIDE The Humpback Whales Educator Guide, created by MacGillivray Freeman Films Educational Foundation in partnership with MacGillivray Freeman Films and Orange County Community Foundation, is appropriate for all intermediate grades (3 to 8) and most useful when used as a companion to the film, but also valuable as a resource on its own. Teachers are strongly encouraged to adapt activities included in this guide to meet the specific needs of the grades they teach and their students. Activities developed for this guide support Next Generation Science Standards (NGSS), Ocean Literacy Principles, National Geography Standards and Common Core Language Arts (see page 27 for a standards alignment chart). An extraordinary journey into the mysterious world of one of nature’s most awe-inspiring marine mammals, Humpback Whales takes audiences to Alaska, Hawaii and the remote islands of Tonga for an immersive look at how these whales communicate, sing, feed, play and take care of their young. Captured for the first time with IMAX® 3D cameras, and found in every ocean on earth, humpbacks were nearly driven to extinction 50 years ago, but today are making a steady recovery. Join a team of researchers as they unlock the secrets of the humpback and find out what makes humpbacks the most acrobatic of all whales, why they sing their haunting songs, and why these 55-foot, 50-ton animals migrate up to 10,000 miles round-trip every year. -

Ocean Giants: Giant Lives 4Th Annual Coastal Discovery Stewardship
4th Coastal Discovery Jan 13 - & Stewardship Feb 28 2017 Annual Celebration FREE movie at Hearst Castle Theater Ocean Giants: Giant Lives Discover the intimate details of the great whales. Every Saturday from January 14 – February 25 at 6pm Starts at 6:45 2/18 and 2/25 The California Central Coast is a spectacular place to watch marine mammals from shore. Visit www.TheWhaleTrail.org to find six viewing locations in Coastal San Luis Obispo County. 40 Must-Do Celebration Activities and Events Visit www.Highway1DiscoveryRoute.com/Stewardship-Travel for dates and times. • 3rd Annual BlendFest on the Coast Wine Event • Central Coast Aquarium Shark Feeding and Tour • Avila Beach Bird Sanctuary & Wildlife Day • Elephant Seal Docent Led Walks • Guadalupe-Nipomo Dunes Natural History Center • Piedres Blancas Light Station Historical Tours VERYROU CO TE. IS CO 1D M Y A W H G I For Lodging Specials and Details go to: H Highway1DiscoveryRoute.com/Stewardship-Travel Wildlife Viewing and Stewardship Tips Visit Highway1DiscoveryRoute.com/Stewardship-Travel Be outside during dawn, dusk, and incoming tides. Birds, fish, and mammals are active during these times. Look for churning water surfaces, diving birds, shiny dolphin backs, seals and otters in bays and on open water. Listen for songbirds singing in bushes and trees, especially during spring and early summer. Be calm and stay awhile. Adopt an unhurried, ‘vacation’ state of mind. A state of relaxed alertness is the best way to see wildlife. Blend in. Animals react to movement. Sit quietly next to a bush or tree and practice the ‘art of invisibility.’ Keep it steady. -

Kiszka Et Al Report V2 Rrr Tc
Intended for IOTC-WPEB 2017 use only – Do not cite without consent from the author Cetacean bycatch in the western Indian Ocean: a review of available information on coastal gillnet, tuna purse seine and pelagic longline fisheries Jeremy J. Kiszka1, Per Berggren2, Gill Braulik3, Tim Collins4, Gianna Minton5 & Randall Reeves6 1 Department of Biological Sciences, Florida International University, North Miami, USA 2 School of Natural & Environmental Sciences, Newcastle University, UK 3 Sea Mammal Research Unit, University of St Andrews, United Kingdom. 4 Megaptera Marine Conservation, Wassenaar, the Netherlands 5 Wildlife Conservation Society, Ocean Giants Program, Bronx, New York, USA 6 Okapi Wildlife Associates, Hudson, Quebec, Canada. Email: [email protected] Abstract Bycatch is the most significant and immediate threat to cetacean populations at the global scale. Here we review available information on cetacean bycatch in industrial and small-scale (mostly artisanal) fisheries in the western Indian Ocean (WIO). In coastal waters of the WIO region, the impact of bycatch has not been quantified in small-scale fisheries, except in bottom-set gillnets and driftnets targeting large pelagic fish (including tuna) off Zanzibar. Based on bycatch data from observer programs and estimated abundance of coastal dolphins, the bycatch off the south coast of Zanzibar was found to be unsustainable. Elsewhere in the region, other species are also bycaught, including coastal, oceanic and migratory species such as humpback whales (Megaptera novaeangliae), mostly in bottom-set and drift gillnets. In open-ocean fisheries, bycatch in pelagic longlines has been recorded but seems to be rare. Species affected are mostly medium-sized delphinids (Globicephala macrorhynchus, Grampus griseus, Pseudorca crassidens) involved in depredation (on either bait or catch). -

WHALES of the ANTARCTIC PENINSULA Science and Conservation for the 21St Century CONTENTS
THIS REPORT HAS BEEN PRODUCED IN COLLABORATION WITH REPORT ANTARCTICA 2018 WHALES OF THE ANTARCTIC PENINSULA Science and Conservation for the 21st Century CONTENTS Infographic: Whales of the Antarctic Peninsula 4 1. PROTECTING OCEAN GIANTS UNDER INCREASING PRESSURES 6 2. WHALES OF THE ANTARCTIC PENINSULA 8 Species found in the Antarctic Peninsula are still recovering from commercial whaling 10 Infographic: Humpback whale migration occurs over multiple international and national jurisdictions 12 Whales face several risks in the region and during migrations 15 Authors: Dr Ari Friedlaender (UC Santa Cruz), Michelle Modest (UC Baleen whales use the Antarctic Peninsula to feed on krill Santa Cruz) and Chris Johnson (WWF Antarctic programme). – the keystone species of the Antarctic food chain 16 Contributors: Infographic: The Western Antarctic Peninsula is critical Dr David Johnston (Duke University), Dr Jennifer Jackson feeding habitat for humpback whales 20 (British Antarctic Survey) and Dr Sarah Davie (WWF-UK). Whales play a critical role in Southern Ocean ecosystems 22 Acknowledgements: Special thanks to Rod Downie (WWF-UK), Dr Reinier Hille Ris Lambers (WWF-NL), Rick Leck (WWF-Aus), 3. NEW SCIENCE IS CHANGING OUR UNDERSTANDING OF WHALES 24 Duke University Marine Robotics and Remote Sensing Lab, California Ocean Alliance and One Ocean Expeditions. Technology is providing scientists and policymakers with data to better understand, monitor and conserve Antarctic whales 26 Graphic Design: Candy Robertson Copyediting: Melanie Scaife Satellite and suction-cup tags uncover whale foraging areas and behaviour 28 Front cover photo: © Michael S. Nolan / Robert Harding Picture Library / National Geographic Creative / WWF Long-Term Ecological Research – Palmer Station, Antarctica 29 Photos taken under research permits include: Dr Ari Drones uncovering a new view from above 30 Friedlaender NMFS 14809, ACA 2016-024 / 2017-034, UCSC IACUC friea1706, and ACUP 4943.