Zootaxa,Five New Species of Cotylean Flatworms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

(Platyhelminthes, Polycladida, Cotylea) from the Persian Gulf, Iran
A peer-reviewed open-access journal ZooKeys 31: 39–51 (2009)First record of the family Pseudocerotidae the Persian Gulf, Iran 39 doi: 10.3897/zookeys.31.136 RESEARCH ARTICLE www.pensoftonline.net/zookeys Launched to accelerate biodiversity research First record of the family Pseudocerotidae (Platyhelminthes, Polycladida, Cotylea) from the Persian Gulf, Iran Zahra Khalili1, Hassan Rahimian2, Jamile Pazooki1 1 University of Shahid Beheshti, G.C, Tehran, Iran 2 University of Tehran, Tehran, Iran Corresponding authors: Hassan Rahimian ([email protected]), Zahra Khalili ([email protected]) Academic editor: E. Neubert, Z. Amr | Received 7 March 2009 | Accepted 14 August 2009 | Published 28 December 2009 Citation: Khalili Z, Rahimian H, Pazooki J (2009) First record of the family Pseudocerotidae (Platyhelminthes, Po- lycladida, Cotylea) from the Persian Gulf, Iran. In: Neubert, E, Amr, Z, Taiti, S, Gümüs, B (Eds) Animal Biodiversity in the Middle East. Proceedings of the First Middle Eastern Biodiversity Congress, Aqaba, Jordan, 20–23 October 2008. ZooKeys 31: 39–51. doi: 10.3897/zookeys.31.136 Abstract In this paper, two species of cotylean Platyhelminthes are recorded for the fi rst time from Qeshm Island, Persian Gulf, Iran. Pictures are taken from living specimens to illustrate shape and colour, and stained sec- tions and drawings are used to describe shape and organisation of some organs. Morphological characters of Persian Gulf specimens of Tytthosoceros lizardensis Newman and Cannon 1996 are compared to those of the type specimens of this species. Keywords Platyhelminthes, new records, Qeshm Island, Persian Gulf, Iran Introduction Most polyclad fl atworms inhabit coral reefs in tropical and subtropical waters, and are espe- cially species-rich throughout the Indo-Pacifi c. -

Platyhelminthes) at the Queensland Museum B.M
VOLUME 53 ME M OIRS OF THE QUEENSLAND MUSEU M BRIS B ANE 30 NOVE mb ER 2007 © Queensland Museum PO Box 3300, South Brisbane 4101, Australia Phone 06 7 3840 7555 Fax 06 7 3846 1226 Email [email protected] Website www.qm.qld.gov.au National Library of Australia card number ISSN 0079-8835 Volume 53 is complete in one part. NOTE Papers published in this volume and in all previous volumes of the Memoirs of the Queensland Museum may be reproduced for scientific research, individual study or other educational purposes. Properly acknowledged quotations may be made but queries regarding the republication of any papers should be addressed to the Editor in Chief. Copies of the journal can be purchased from the Queensland Museum Shop. A Guide to Authors is displayed at the Queensland Museum web site www.qm.qld.gov.au/organisation/publications/memoirs/guidetoauthors.pdf A Queensland Government Project Typeset at the Queensland Museum THE STUDY OF TURBELLARIANS (PLATYHELMINTHES) AT THE QUEENSLAND MUSEUM B.M. ANGUS Angus, B.M. 2007 11 30: The study of turbellarians (Platyhelminthes) at the Queensland Museum. Memoirs of the Queensland Museum 53(1): 157-185. Brisbane. ISSN 0079-8835. Turbellarian research was largely ignored in Australia, apart from some early interest at the turn of the 19th century. The modern study of this mostly free-living branch of the phylum Platyhelminthes was led by Lester R.G. Cannon of the Queensland Museum. A background to the study of turbellarians is given particularly as it relates to the efforts of Cannon on symbiotic fauna, and his encouragement of visiting specialists and students. -

(Platyhelminthes, Turbellaria, Polycladida) from Karachi Coast
International Journal of Research Studies in Zoology (IJRSZ) Volume 2, Issue 2, 2016, PP 23-28 ISSN 2454-941X http://dx.doi.org/10.20431/2454-941X.0202005 www.arcjournals.org Short Notes on Marine Polycladids (Platyhelminthes, Turbellaria, Polycladida) from Karachi Coast Quddusi B. Kazmi Marine Reference Collection and Resource Centre, University of Karachi, Pakistan Abstract: Ten new records of marine polycladid worms are subject of the present notes from Pakistan. Each species is photographed and discussed briefly. 1. INTRODUCTION The Polycladida represents a highly diverse clade of free-living marine turbellarian flatworms. They are known from the littoral to the sub littoral zone. Although not related to molluscs, they are often mistaken for sea slugs because of their brilliant colour patterns. There is little known about the biodiversity of polycladid flatworms from the Indian Ocean. In Pakistan, studies on polycladids have remained neglected, first report was by Kazmi (1996), then Fatima and Barkati (1999) as Stylochoplanapallida reported Emprosthopharynxpallida (Quatrefage,1845) and latelyKazmi and Naushaba (2013) listed 4 unidentified species or only identified to genus level, of these , their unspecified genus Pseudocerosis now identified as belonging to Pseudocerossusanae Newman and Anderson ,1997 ,an undetermined pseudocertid is now named as Tytthosoceroslizardensis Newman and Cannon,1996 and another undetermined genus is given as Cestoplanarubrocinta (Grube, 1840) ,more species are added here;all are briefly described here -

Neotype Designation for the Marine Flatworm, Acanthozoon Alderi
Zoological Studies 57: 45 (2018) doi:10.6620/ZS.2018.57-45 Open Access Neotype Designation for the Marine Flatworm, Acanthozoon alderi (Polycladida: Cotylea: Pseudocerotidae), from India with Comments on the Taxonomical Status of the Genus Sudhanshu Dixit1, Verónica N. Bulnes2,*, and Chelladurai Raghunathan3 1Centre for Marine Living Resources and Ecology, Kochi, India. E-mail: [email protected] 2Laboratorio de Zoología de Invertebrados I. INBIOSUR-CONICET-UNS. San Juan 670. 8000 Bahía Blanca. Buenos Aires. Argentina 3Zoological Survey of India, M - Block, Alipore, Kolkata, India. E-mail: [email protected] (Received 16 April 2018; Accepted 16 August 2018; Published 27 September 2018; Communicated by Benny K.K. Chan) Citation: Dixit S, Bulnes VN, Raghunathan C. 2018. Neotype designation for the marine flatworm, Acanthozoon alderi (Polycladida: Cotylea: Pseudocerotidae), from India with comments on the taxonomical status of the genus. Zool Stud 57:45. doi:10.6620/ZS.2018.57- 45. Sudhanshu Dixit, Verónica N. Bulnes, and Chelladurai Raghunathan (2018) Acanthozoon alderi is an ovoid, medium-sized pseudocerotid. Body margin ruffled; pseudotentacles black and pointed, with white tips. Dorsal surface covered with papillae, except for the cerebral region. Background colour light brown, with marbled blackish pattern, middorsal black band with white blotches; black submarginal band and marginal white rim. This species was described from Borneo; however, no type specimen was designated or deposited in any museum by the author. Many nomenclature problems and misidentification have been encountered with this species (it has been identified as Acanthozoon sp. in many instances). Thus, it is necessary to designate a neotype to solve the problems of doubtful and confusing identities and maintain nomenclature stability. -

Platyhelminthes: Polycladida) in Botany Bay, New South Wales, Australia
TAXONOMY AND ECOLOGY OF PREDATORY MARINE FLATWORMS (PLATYHELMINTHES: POLYCLADIDA) IN BOTANY BAY, NEW SOUTH WALES, AUSTRALIA by Ka-Man Lee A thesis submitted in fulfilment of the requirements for the degree of Master of Science by research University of New South Wales April 2006 ORIGINALITY STATEMENT ‘I hereby declare that this submission is my own work and to the best of my knowledge it contains no materials previously published or written by another person, or substantial proportions of material which have been accepted for the award of any other degree or diploma at UNSW or any other educational institution, except where due acknowledgement is made in the thesis. Any contribution made to the research by others, with whom I have worked at UNSW or elsewhere, is explicitly acknowledged in the thesis. I also declare that the intellectual content of this thesis is the product of my own work, except to the extent that assistance from others in the project’s design and conception or in style, presentation and linguistic expression is acknowledged.’ Signed Ka-Man Lee April 2006 II ACKNOWLEDGEMENTS Without the encouragement and enthusiasm of my supervisor, Dr. Emma Johnston, this thesis would not have been possible. Thank you for allowing me to pursue some innovative experiments and for your inspiration and criticism along the way. I thoroughly appreciated your patience and guidance. I am eternally grateful to my co-supervisors, Assoc. Prof A. Michel Beal and Dr. Alistair Poore. Assoc. Prof Michel Beal has been incredibly supportive and generous with his time. I thoroughly enjoyed and appreciated your endless supply of patience and guidance. -

Marine-Flatworms-Of-The-Tropical-Indo-Pacific-Look
Marine Flatworms of the Tropical Indo-Pacific Photographic guide on marine polyclads with 580+ species Andrey Ryanskiy PICTORIAL INDEX TO POLYCLAD FAMILIES AND GENERA PSEUDOCEROTIDAE: ACANTHOZOON - 9 THYSANOZOON - 14 BULACEROS - 16 MAIAZOON - 17 NYMPHOZOON - 17 PHRIKOCEROS - 18 PSEUDOBICEROS - 21 PSEUDOCEROS - 38 EURYLEPTIDAE: CYCLOPORUS - 86 EURYLEPTA - 92 EURYLEPTID - 101 STYLOSTOMUM - 101 DIPOSTHIDAE: PROSTHIOSTOMIDAE: MARITIGRELLA - 102 PROSTHECERAEUS - 105 PERICELIS - 106 ENCHIRIDIUM - 107 CESTOPLANIDAE: BONINIIDAE: LURYMARE - 107 PROSTHIOSTOMUM - 112 CESTOPLANA - 112 BONINIA - 113 PLANOCERIDAE: CALLIOPLANIDAE: PARAPLANOCERA - 115 PLANOCERA - 120 CALLIOPLANA - 121 HOPLOPLANIDAE - 122 STYLOCHIDAE: GNESIOCEROTIDAE: LIMNOSTYLOCHIDAE - 122 ILYELLA - 123 STYLOCHID - 123 ECHINOPLANA - 126 NOTOPLANIDAE: UNIDENTIFIED ACOTYLEANS3 GNESIOCEROS - 126 NOTOPLANA - 127 LEPTOPLANIDAE - 128 - 129 FLATWORMS: BASIC KNOWLEDGE Why are they flat? Polyclads are considered the most primitive bilaterally symmetrical animals (left side mirrors the right). They evolved from hydra-like animals about 550 million years ago. Flatworms have no body cavity other than the gut. Respiratory and blood vessel systems are completely missing and diffusion is used for transport of oxygen inside the body. This constrains flatworms to be flat as possible for maintaining metabolism, since no cell can be too far from the outside, making a flattened body shape necessary. How do they eat? Flatworms/Polyclads have a mouth with pharynx inside: a muscular tube through which the flatworm can suck food. Pharynx may be tubular or ruffled with numerous folds (more details on External Morphology Basics pages) Flatworms are carnivorous, feeding on small invertebrates, suctioning entirely their prey or digesting a part of it. Many species of the Pseudocerotidae family prefer ascidians, sponges, and bryozoans. For feeding, the pharynx protrudes and can be expanded into the individual zooids of colonial ascidians. -
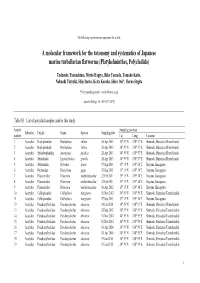
Platyhelminthes, Polycladida)
The following supplement accompanies the article A molecular framework for the taxonomy and systematics of Japanese marine turbellarian flatworms (Platyhelminthes, Polycladida) Tadasuke Tsunashima, Morio Hagiya, Riko Yamada, Tomoko Koito, Nobuaki Tsuyuki, Shin Izawa, Keita Kosoba, Shiro Itoi*, Haruo Sugita *Corresponding author: [email protected] Aquatic Biology 26: 159–167 (2017) Table S1. List of polyclad samples used in this study Sample Sampling location Suborder Family Genus Species Sampling date number Lat. Long. Location 1 Acotylea Hoploplanidae Hoploplana villosa 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 2 Acotylea Hoploplanidae Hoploplana villosa 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 3 Acotylea Stylochoplanidae Amemiyaia pacifica 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 4 Acotylea Stylochidae Leptostylochus gracilis 26 Apr 2013 34° 39' N 138° 57' E Shimoda, Shizuoka (Ebisu Island) 5 Acotylea Stylochidae Stylochus ijimai 07 Sep 2013 35° 15' N 139° 34' E Hayama, Kanagawa 6 Acotylea Ilyplanidae Discoplana gigas 07 Sep 2013 35° 15' N 139° 34' E Hayama, Kanagawa 7 Acotylea Planoceridae Planocera multitentaculata 22 Feb 2011 35° 15' N 139° 34' E Hayama, Kanagawa 8 Acotylea Planoceridae Planocera multitentaculata 22 Feb 2011 35° 15' N 139° 34' E Hayama, Kanagawa 9 Acotylea Planoceridae Planocera multitentaculata 06 Apr 2012 35° 15' N 139° 34' E Hayama, Kanagawa 10 Acotylea Callioplanidae Callioplana marginata 01 Nov 2013 34° 39' N 138° 59' E Shimoda, Shizuoka -

Animal Phylogeny and the Ancestry of Bilaterians: Inferences from Morphology and 18S Rdna Gene Sequences
EVOLUTION & DEVELOPMENT 3:3, 170–205 (2001) Animal phylogeny and the ancestry of bilaterians: inferences from morphology and 18S rDNA gene sequences Kevin J. Peterson and Douglas J. Eernisse* Department of Biological Sciences, Dartmouth College, Hanover NH 03755, USA; and *Department of Biological Science, California State University, Fullerton CA 92834-6850, USA *Author for correspondence (email: [email protected]) SUMMARY Insight into the origin and early evolution of the and protostomes, with ctenophores the bilaterian sister- animal phyla requires an understanding of how animal group, whereas 18S rDNA suggests that the root is within the groups are related to one another. Thus, we set out to explore Lophotrochozoa with acoel flatworms and gnathostomulids animal phylogeny by analyzing with maximum parsimony 138 as basal bilaterians, and with cnidarians the bilaterian sister- morphological characters from 40 metazoan groups, and 304 group. We suggest that this basal position of acoels and gna- 18S rDNA sequences, both separately and together. Both thostomulids is artifactal because for 1000 replicate phyloge- types of data agree that arthropods are not closely related to netic analyses with one random sequence as outgroup, the annelids: the former group with nematodes and other molting majority root with an acoel flatworm or gnathostomulid as the animals (Ecdysozoa), and the latter group with molluscs and basal ingroup lineage. When these problematic taxa are elim- other taxa with spiral cleavage. Furthermore, neither brachi- inated from the matrix, the combined analysis suggests that opods nor chaetognaths group with deuterostomes; brachiopods the root lies between the deuterostomes and protostomes, are allied with the molluscs and annelids (Lophotrochozoa), and Ctenophora is the bilaterian sister-group. -

Informe De Planarias Y Ascarias
UNIVERSIDAD PRIVADA DE TACNA FACULTAD DE INGENIERÍA ESCUELA PROFESIONAL DE INGENIERÍA AMBIENTAL PLANARIAS Y ASCARIAS ALUMNA : Mariela Alejandra Cutipa Vargas CÓDIGO : 2013046501 ASIGNATURA : Zoología DOCENTE : MSc.Blgo.Mlgo.Richard Sabino Lazo Ramos CICLO : III TACNA – PERÚ 2014 CONTENIDO 0 1. PLANARIAS 1 Pseudobiceros gloriosus 2 Pseudoceros Dimidiatus 2 Pseudoceros ferrugineus 3 Pseudobiceros bifurcus 3 2. ASCARIAS 11 CONCLUSIONES 19 1. PLANARIAS INTRODUCCION: La Turbellaria (conocido también como planaria o gusano plano) es un animal bentónico, es decir, que vive en las profundidades. Particularmente se le puede clasificar como bentónico libre (puede vivir tanto en agua de mar o en agua dulce). Mide entre 1 a 60 centímetros de longitud. Su alimentación es de tipo carnívora o necrófaga. Su forma de desplazamiento es por medio de microvellosidades invisibles al ojo humano denominadas cilios. 1 1.1.CARACTERISTICAS (Moreno, 2014) Los turbelarios (Turbellaria), conocidos vulgarmente como planarias, son una clase del filo platelmintos ("gusanos planos") de vida libre y de pequeño tamaño. Acoela y Nemertodermatida, que habían sido considerados turbelarios, se clasifican actualmente en un filo separado (Acoelomorpha). La mayoría son organismos bentónicos, marinos o de agua dulce; otros han dejado este medio para adaptarse a terrenos húmedos. Su locomoción depende de cilios, y excavan activamente en busca de comida. La mayoría son carnívoros. La planarias Dugesia es un turbelario representativo. Existen unas 3000 especies; hermafroditas. Entre 5 y 55 mm de longitud; algunas especies tropicales alcanzan 50 cm. Coloración variable. Pseudoceros Pseudobiceros Dimidiatus gloriosus 2 Pseudobiceros bifurcus Pseudoceros ferrugineus Bipalium 1.2.PLANO DE SIMETRIA: FIG.1-PLANO DE SIMETRIA DE UNA TURBELLARIA 1.3.PARTES DE UNA TURBELLARIA : 3 FIG.2-PARTES DE UNA TURBELLARIA PARED DEL CUERPO: Epitelio: Carece de cutícula y es celular. -

Pseudoceros Astrorum, a New Species of Polycladida (Cotylea, Pseudocerotidae) from Northeastern Brazil
Zootaxa 3881 (1): 094–100 ISSN 1175-5326 (print edition) www.mapress.com/zootaxa/ Article ZOOTAXA Copyright © 2014 Magnolia Press ISSN 1175-5334 (online edition) http://dx.doi.org/10.11646/zootaxa.3881.1.7 http://zoobank.org/urn:lsid:zoobank.org:pub:9C0FB9F8-C565-4627-87A2-D44FDFAB6781 Pseudoceros astrorum, a new species of Polycladida (Cotylea, Pseudocerotidae) from Northeastern Brazil VERONICA N. BULNES1 & YAN TORRES2 1Zoología de Invertebrados I, Universidad Nacional del Sur. San Juan 670. 8000 Bahía Blanca, Argentina 2Departamento de Biologia, Centro de Ciências, Universidade Federal do Ceará, Ceará, Brasil Abstract Pseudoceros astrorum n. sp. is characterized by a smooth dorsal surface with a brown ground colour, and with net-like pattern of small black granules, white spots of different sizes uniformly distributed, a thin black sub-marginal band, and a white marginal rim. The pseudotentacles are dark brown with white tips and the anterior margin and cerebral region is devoid of pigmentation. The male system is characterised by conspicuous spermiducal bulbs, a conical curved penis stylet, and the sucker lies more or less posterior. With this contribution, the number of known species from Brazil is now 72, and has created new interest in the lesser-known polyclad fauna from the northeast coast of Brazil. Key words: biodiversity, morphology, Marine flatworms, Western Atlantic, Cotylea Introduction The Pseudocerotidae is one of the largest families within the Cotylea, with a large number of described species. The phylogeny as well as the monophyly of some genera are still unclear (Rawlinson & Litvaitis 2008). Pseudoceros is one of the most common tropical polyclad genera, and species of Pseudoceros are determined on the basis of their colour patterns (Newman & Cannon 1995), external morphological features, as well as the reproductive biology, and microanatomy (Hyman 1951; Prudhoe 1985; Faubel 1984; Bolaños et al. -

Marine Biodiversity in India
MARINEMARINE BIODIVERSITYBIODIVERSITY ININ INDIAINDIA MARINE BIODIVERSITY IN INDIA Venkataraman K, Raghunathan C, Raghuraman R, Sreeraj CR Zoological Survey of India CITATION Venkataraman K, Raghunathan C, Raghuraman R, Sreeraj CR; 2012. Marine Biodiversity : 1-164 (Published by the Director, Zool. Surv. India, Kolkata) Published : May, 2012 ISBN 978-81-8171-307-0 © Govt. of India, 2012 Printing of Publication Supported by NBA Published at the Publication Division by the Director, Zoological Survey of India, M-Block, New Alipore, Kolkata-700 053 Printed at Calcutta Repro Graphics, Kolkata-700 006. ht³[eg siJ rJrJ";t Œtr"fUhK NATIONAL BIODIVERSITY AUTHORITY Cth;Govt. ofmhfUth India ztp. ctÖtf]UíK rvmwvtxe yÆgG Dr. Balakrishna Pisupati Chairman FOREWORD The marine ecosystem is home to the richest and most diverse faunal and floral communities. India has a coastline of 8,118 km, with an exclusive economic zone (EEZ) of 2.02 million sq km and a continental shelf area of 468,000 sq km, spread across 10 coastal States and seven Union Territories, including the islands of Andaman and Nicobar and Lakshadweep. Indian coastal waters are extremely diverse attributing to the geomorphologic and climatic variations along the coast. The coastal and marine habitat includes near shore, gulf waters, creeks, tidal flats, mud flats, coastal dunes, mangroves, marshes, wetlands, seaweed and seagrass beds, deltaic plains, estuaries, lagoons and coral reefs. There are four major coral reef areas in India-along the coasts of the Andaman and Nicobar group of islands, the Lakshadweep group of islands, the Gulf of Mannar and the Gulf of Kachchh . The Andaman and Nicobar group is the richest in terms of diversity. -
A New Species and New Records of Pseudoceros (Turbellaria, Polycladida) from the Maldives
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/266225294 A new species and new records of Pseudoceros (Turbellaria, Polycladida) from the Maldives Article CITATIONS READS 3 531 2 authors, including: R Charles Anderson Manta Marine Pvt Ltd 67 PUBLICATIONS 1,406 CITATIONS SEE PROFILE All content following this page was uploaded by R Charles Anderson on 15 January 2015. The user has requested enhancement of the downloaded file. J. South Asian nat. Hist, ISSN 1022-0828. March, 1997. Vol. 2, No. 2; pp. 247-256; 7 figs. © Wildlife Heritage Trust of Sri Lanka, 95 Cotta Road, Colombo 8, Sri Lanka. A new species and new records of Pseudoceros (Turbellaria, Polycladida) from the Maldives Leslie J. Newman* and R. Charles Anderson** Abstract Anew species of brightly coloured pseudocerotid fLatworm, Pseudoceros susanae sp. nov., is described from the Maldives. This species is diagnosed by its unique colour pattern: blue background, orange longitudinal stripe bifurcated by a narrow white line and bright purple-red margin. New records are also given for P. goslineri Newman & Cannon, 1994 and P. scintillatus Newman & Cannon, 1994. Key words: Polycladida, Pseudocerotidae, Pseudoceros, flatworms, taxonomy, Maldives Introduction Pseudocerotid polyclad flatworms are often conspicuous inhabitants of coral reefs. They are commonly found on the reef slope or under rubble feeding on small invertebrates such as colonial ascidians (Newman & Cannon, 1994a). The biodiversity of the more colourful pseudocerotids is known to be high in Pacific waters with over 130 species recorded from just one location on the Great Barrier Reef, Australia (Newman & Cannon, 1994a, b).