P. 1 Cop15 Prop. 7 CONVENTION on INTERNATIONAL TRADE
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

America&Apos;S Unknown Avifauna: the Birds of the Mariana Islands
ß ß that time have been the basis for con- America's unknown avifauna. siderable concern (Vincent, 1967) and indeed appear to be the basis for the the birds of inclusion of several Mariana birds in the U.S. Fish & Wildlife Service (1976) list of the Mariana Islands Endangered Species.These brief war- time observationswere important, but no significant investigationshave been conductedin the ensuingthirty yearsto "Probably no otherAmerican birds determine the extent to which the are aspoorly known as these." endemic avifauna of these islands may haverecovered. Importantly, no assess- mentshave been made of the impactof H. Douglas Pratt, Phillip L. Bruner the military's aerial planting of the exoticscrubby tree known as tangan- and Delwyn G. Berrett tangan, Leucaenaglauca, to promote revegetationafter the war. This 'treeis known as "koa haole" in Hawaii. restricted both in their time for bird ß ß announcesthe signthat greets observation and in their movements on v•sitors to Guam. Few Americans realize the islands. Their studies were made in authorsURING THEvisitedSUMMER the islandsOF1076the of that the nation's westernmost territories 1945 and 1946 when most of the Mari- Saipan,Tinian, Rota, and Guam, and m he across the International Date Line in anaswere just beginningto recoverfrom 1978 Bruner and Pratt returned to Sai- the far westernPacific. Guam, the larg- the ravagesof war (Baker, 1946).Never- pan and Guam. We havespent a total of est and southernmost of the Mariana theless, population estimates made at 38 man/dayson Saipan,four on Tinian, Islands,has been a United Statesposses- s•on since Spain surrendered her sov- & Agrihan ereigntyover the island at the end of the Sparash-AmericanWar. -
Common Name Spring Summer Fall Winter Greater White-Fronted Goose
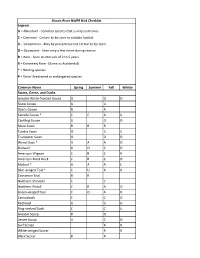
Illinois River NWFR Bird Checklist Legend: A = Abundant - Common species that is very numerous C = Common - Certain to be seen in suitable habitat U = Uncommon - May be present but not certain to be seen O = Occasional - Seen only a few times during season R = Rare - Seen at intervals of 2 to 5 years X = Extremely Rare - (Same as Accidental) * = Nesting species # = State threatened or endangered species Common Name Spring Summer Fall Winter Swans, Geese, and Ducks Greater White-fronted Goose O O O Snow Goose U U Ross's Goose R R Canada Goose * C C A U Cackling Goose U O O Mute Swan R R R Tundra Swan O U U Trumpeter Swan O O O Wood Duck * A A A O Gadwall U O C O American Wigeon C R C R American Black Duck C R C O Mallard * A A A C Blue-winged Teal * C U A R Cinnamon Teal R R Northern Shoveler C C Northern Pintail C R A O Green-winged Teal C O A R Canvasback C C O Redhead U U O Ring-necked Duck C C U Greater Scaup R O Lesser Scaup A C O Surf Scoter R R White-winged Scoter R R Black Scoter R R Long-tailed Duck R R Bufflehead U U O Common Goldeneye U U U Hooded Merganser * C O C O Common Merganser C O C Red-breasted Merganser O O Ruddy Duck C R C U Upland Game Birds Ring-necked Pheasant O O O O Wild Turkey * O O O O Northern Bobwhite * U U U U Loons, Grebes, Pelicans, and Cormorants Red-throated Loon R Common Loon O O Pied-billed Grebe * C O U Horned Grebe U U Eared Grebe R R Western Grebe R American White Pelican A A O Double-crested Cormorant A O A O Bitterns, Herons, and Vultures American Bittern # R R R Least Bittern * # R R R Great Blue -

( 7)-Revista Ac. Nº 84
Ardeol a 67(2), 2020, 449 -494 DOI: 10.13157/arla.67.2.2020.on NOTICIARIO ORNITOLÓGICO ORNITHOLOGICAL NEWS 1 2 3 Blas MOLIN A , Javier PRIET A , Juan Antonio LORENZ O (Canarias) 4 y Carlos LÓPEZ -JURAD O (Baleares) RESUMEN .— En este informe se muestra información sobre 149 especies que se reparten por toda la geografía nacional. Entre ellas, se incluyen 28 especies que aparecen de forma escasa para las que se muestra su distribución espacio-temporal en 2019 mediante mapas y gráficas (véase De Juana y Garcia, 2015; Gil-Velasco et al ., 2018; Rouco et al ., 2018). Para contabilizar el número de citas para cada espe- cie escasa se revisaron las observaciones disponibles en diferentes plataformas de recogida de datos, especialmente en eBird ( eBird , 2020; Observado , 2020; Reservoir Birds , 2020 y Ornitho , 2020), des - cartando aquellas que aparentemente correspondieran a las mismas citas hechas por diferentes observa - dores con el objeto de contar con muestras de datos lo suficientemente homogéneas. Para el archipiélago canario, se incorpora un buen número de citas correspondientes al episodio de calima que se produjo a finales de febrero de 2020. Dichas circunstancias meteorológicas provocaron la arribada de muchas aves accidentales procedentes del noroeste africano de las que dará cuenta el correspondiente informe del Comité de Rarezas de SEO/BirdLife, junto con muchas otras especies de paso que se pudieron observar en las islas con mayor frecuencia y en números más altos de lo habitual, y de las que se sinte- tiza en el presente informe aquellas observaciones más relevantes. Se sigue la secuencia taxonómica de acuerdo con la nueva lista patrón de las aves de España (Rouco et al ., 2019). -

European Red List of Birds
European Red List of Birds Compiled by BirdLife International Published by the European Commission. opinion whatsoever on the part of the European Commission or BirdLife International concerning the legal status of any country, Citation: Publications of the European Communities. Design and layout by: Imre Sebestyén jr. / UNITgraphics.com Printed by: Pannónia Nyomda Picture credits on cover page: Fratercula arctica to continue into the future. © Ondrej Pelánek All photographs used in this publication remain the property of the original copyright holder (see individual captions for details). Photographs should not be reproduced or used in other contexts without written permission from the copyright holder. Available from: to your questions about the European Union Freephone number (*): 00 800 6 7 8 9 10 11 (*) Certain mobile telephone operators do not allow access to 00 800 numbers or these calls may be billed Published by the European Commission. A great deal of additional information on the European Union is available on the Internet. It can be accessed through the Europa server (http://europa.eu). Cataloguing data can be found at the end of this publication. ISBN: 978-92-79-47450-7 DOI: 10.2779/975810 © European Union, 2015 Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale or other commercial purposes is prohibited without prior written permission of the copyright holder. Printed in Hungary. European Red List of Birds Consortium iii Table of contents Acknowledgements ...................................................................................................................................................1 Executive summary ...................................................................................................................................................5 1. -

Sauvie Island Bird Checklist Documents
WATERFOWL S S F W Cooper’s Hawk* O O O O Pectoral Sandpiper O Northern Goshawk R R Sharp-tailed Sandpiper A Tundra Swan U R U C Red-shouldered Hawk A Stilt Sandpiper A Trumpeter Swan R R R R Red-tailed Hawk* C C C C Buff-breasted Sandpiper A Greater White-fronted Goose U R U O Swainson’s Hawk A A Ruff A A Snow Goose O O U Rough-legged Hawk O O U Short-billed Dowitcher U Ross’s Goose R Long-billed Dowitcher U U U O Ferruginous Hawk A A Emperor Goose R R American Kestrel* C C C C Common Snipe* U O U C Canada Goose* C U C C Merlin O O O O Wilson’s Phalarope O R O SYMBOLS Brant O O O Prairie Falcon R R R R Red-necked Phalarope O R O S - March - May Wood Duck* C C U U Peregrine Falcon # O O O Red Phalarope A A A S - June - August Mallard* C C C C Gyrfalcon A F - September - November American Black Duck A GULLS & TERNS S S F W W - December - February Gadwall* U O U U GALLINACEOUS BIRDS S S F W # - Threatened or Endangered Species Green-winged Teal C U C C Parasitic Jaeger A * - Breeds Locally American Wigeon C U C C Ring-necked Pheasant* U O U U Franklin’s Gull A A A A Eurasian Wigeon O O O Ruffed Grouse* O O O O Bonaparte’s Gull O R O R C - Common U - Uncommon O - Occasional Northern Pintail* C U C C California Quail* R R R R Ring-billed Gull C U U C R - Rare A - Accidental Northern Shoveler* C O C C Mew Gull U O O C Blue-winged Teal* R R R R RAILS, COOTS & CRANES S S F W California Gull C O U C LOONS & GREBES S S F W Cinnamon Teal* U C U O Herring Gull U O U Canvasback O O O Virginia Rail* -

Section 3.6 Marine Birds
3.6 Marine Birds MARIANA ISLANDS TRAINING AND TESTING FINAL EIS/OEIS MAY 2015 TABLE OF CONTENTS 3.6 MARINE BIRDS .................................................................................................................... 3.6-1 3.6.1 INTRODUCTION .............................................................................................................................. 3.6-1 3.6.1.1 Endangered Species Act ............................................................................................................. 3.6-2 3.6.1.2 Migratory Bird Treaty Act Species and 50 Code of Federal Regulations Part 21.15 Requirements ............................................................................................................................. 3.6-3 3.6.1.3 United States Fish and Wildlife Service Birds of Conservation Concern ................................... 3.6-4 3.6.1.4 Major Bird Groups...................................................................................................................... 3.6-4 3.6.1.5 Areas Included in the Analysis ................................................................................................... 3.6-6 3.6.2 AFFECTED ENVIRONMENT ................................................................................................................ 3.6-7 3.6.2.1 Group Size .................................................................................................................................. 3.6-8 3.6.2.2 Diving ........................................................................................................................................ -

Moorestown Township Environmental Resource Inventory
APPENDIX C Vertebrate Animals Known or Probable in Moorestown Township Mammals Common Name Scientific Name Status Opossum Didelphis marsupialis Stable Eastern Mole Scalopus aquaticus Stable Big Brown Bat Eptesicus fuscus Stable Little Brown Bat Myotis lucifugus Stable Eastern Cottontail Sylvilagus floridanus Stable Eastern Chipmunk Tamias striatus Stable Gray Squirrel Sciurus carolinensis Stable White-footed Mouse Peromyscus leucopus Stable Meadow Vole Microtus pennsylvanicus Stable Muskrat Ondatra zibethicus Stable Pine Vole Microtus pinetorum Stable Red Fox Vulpes vulpes Stable Gray Fox Urocyon cinereoargenteus Stable Raccoon Procyon lotor Stable Striped Skunk Mephitis mephitis Stable River Otter Lutra canadensis Stable Beaver Castor candensis Increasing White-tailed Deer Odocoileus virginianus Decreasing Source: NJDEP, 2012 C-1 Birds Common Name Scientific Name NJ State Status Loons - Grebes Pied-Billed Grebe Podilymbus podiceps E Gannets - Pelicans - Cormorants Double Crested Cormorant Phalacrocorax auritus S Bitterns - Herons - Ibises American Bittern Botaurus lentiginosus E Least Bittern Ixobrychus exilis SC Black Crowned Night Heron Nycticorax nycticorax T Green Heron Butorides virescens RP Great Blue Heron Ardea herodias SC Great Egret Ardea alba RP Geese - Swans - Ducks Canada Goose Branta canadensis INC Snow Goose Chen caerulescens INC American Wigeon Anas americana S Common Merganser Mergus merganser S Hooded Merganser Lophodytes cucullatus S Green-winged Teal Anas carolinensis RP Mallard Anas platyrhynchos INC Northern Pintail -

Blue-Winged and Green-Winged Teals, Are the Marshes, Swamps, and Ponds of Zero to Low Water Movement
BirdWalk Newsletter 1.29.2017 Walk Conducted by: Perry Nugent Newsletter Written by: Jayne J. Matney Photo right by Cary McDonald Blue-winged Teal male with duckweed beak and chest followed by female partner Photo below by Chuck Fuhrman Two Green-winged Teal males This week will be the first in a series of articles covering the ducks of Magnolia Plantation. Most of our duck population is not of permanent residences. Instead, they migrate in for wintering and migrate out for breeding. The males are called “drakes” and the females are called “hens”. Some of these ducks are considered “dabblers”, which means they eat primarily along the surface of the water chewing or filtering from the surface and rarely dive, while others are the “divers”, which do just that-they dive head first into the water for feeding. Dabbling ducks will occasionally dive for food or to escape predators. Photo above left by Chuck Fuhrman Blue-winged Teal Photo above right by Perry Nugent Green-winged Teal The Blue-winged Teal, Anas discors, (above left) and the Green-winged Teal, Anas crecca, (above right) will be discussed this week. They are in the category of dabblers; both species primarily feed off of aquatic plants and seeds from the surface of the water and small larvae, insects, and crustaceans that may be found there as well. Apparently, egg laying females may feed mostly on animal rather than plant during those special times. Both species are small comparatively to other types of ducks, rest out of the water on stumps, rocks and broken branches, and are fast in flight. -
![Ciconiiformes, Charadriiformes, Coraciiformes, and Passeriformes.]](https://docslib.b-cdn.net/cover/0282/ciconiiformes-charadriiformes-coraciiformes-and-passeriformes-1100282.webp)
Ciconiiformes, Charadriiformes, Coraciiformes, and Passeriformes.]
Die Vogelwarte 39, 1997: 131-140 Clues to the Migratory Routes of the Eastern Fly way of the Western Palearctics - Ringing Recoveries at Eilat, Israel [I - Ciconiiformes, Charadriiformes, Coraciiformes, and Passeriformes.] By Reuven Yosef Abstract: R euven , Y. (1997): Clues to the Migratory Routes of the Eastern Fly way of the Western Palearctics - Ringing Recoveries at Eilat, Israel [I - Ciconiiformes, Charadriiformes, Coraciiformes, and Passeriformes.] Vogelwarte 39: 131-140. Eilat, located in front of (in autumn) or behind (in spring) the Sinai and Sahara desert crossings, is central to the biannual migration of Eurasian birds. A total of 113 birds of 21 species ringed in Europe were recovered either at Eilat (44 birds of 12 species) or were ringed in Eilat and recovered outside Israel (69 birds of 16 spe cies). The most common species recovered are Lesser Whitethroat {Sylvia curruca), White Stork (Ciconia cico- nia), Chiffchaff (Phylloscopus collybita), Swallow {Hirundo rustica) Blackcap (S. atricapilla), Pied Wagtail {Motacilla alba) and Sand Martin {Riparia riparia). The importance of Eilat as a central point on the migratory route is substantiated by the fact that although the number of ringing stations in eastern Europe and Africa are limited, and non-existent in Asia, several tens of birds have been recovered in the past four decades. This also stresses the importance of taking a continental perspective to future conservation efforts. Key words: ringing, recoveries, Eilat, Eurasia, Africa. Address: International Bird Center, P. O. Box 774, Eilat 88106, Israel. 1. Introduction Israel is the only land brigde between three continents and a junction for birds migrating south be tween Europe and Asia to Africa in autumn and north to their breeding grounds in spring (Yom-Tov 1988). -

Ibastoryspring08.Pdf
irds find Maine attractive for many of the same reasons we do—the state offers a unique blend of landscapes spanning from mountains to the sea, with forests, grasslands, rivers, marshes, and long coastlines in between. B Where we find beautiful places to hike and kayak, camp and relax, birds find the habitat they need for their survival. But while Maine’s diverse habitats serve an important role for over IBAs 400 bird species—some threatened, endangered, or of regional conservation in concern—the state’s not immune to a growing list of threats that puts these birds at further risk. Habitat loss, degradation, and fragmentation due to development, toxins such as mercury and lead, oil spills on the coast and Maine inland waters, and climate change are top among them. BY ANDREW COLVIN In the face of these threats, a crucial step in conserving Maine’s birds is to identify the areas of the state that are most important for breeding, wintering, and migration. After several years of working toward that goal, Maine Audubon Lists Maine Audubon has recently completed the first phase of its Important 22 of the Most Important Bird Areas (IBA) program, identifying 22 areas across Maine that are vital Places in Maine for Vulnerable Birds to state—and even global—bird populations. HANS TOOM ERIC HYNES Eight of the rare birds used to identify IBAs in Maine (clockwise from left): Short-eared owl, black-throated blue warbler, least tern, common moorhen, scarlet tanager, harlequin duck, saltmarsh sharp-tailed sparrow, and razorbill. MIKE FAHEY Important -
Bird List IBA
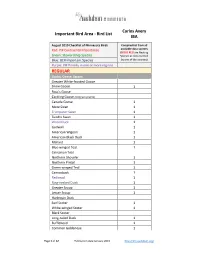
Carlos Avery Important Bird Area - Bird List IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented Blue: BCR Important Species by one of the sources) Purple: PIF Priority in one or more regions REGULAR Ducks, Geese, Swans Greater White-fronted Goose Snow Goose 1 Ross's Goose Cackling Goose (tallgrass prairie) Canada Goose 1 Mute Swan 1 Trumpeter Swan 1 Tundra Swan 1 Wood Duck 1 Gadwall 1 American Wigeon 1 American Black Duck 1 Mallard 1 Blue-winged Teal 1 Cinnamon Teal Northern Shoveler 1 Northern Pintail 1 Green-winged Teal 1 Canvasback 1 Redhead 1 Ring-necked Duck 1 Greater Scaup 1 Lesser Scaup 1 Harlequin Duck Surf Scoter 1 White-winged Scoter 1 Black Scoter Long-tailed Duck 1 Bufflehead 1 Common Goldeneye 1 Page 1 of 12 Publication date January 2015 http://mn.audubon.org/ Carlos Avery Important Bird Area - Bird List IBA August 2010 Checklist of Minnesota Birds Compiled list from all Red: PIF Continental Importance available data sources (BOLD RED are Nesting Green: Stewardship Species Species as documented Blue: BCR Important Species by one of the sources) Purple: PIF Priority in one or more regions Hooded Merganser 1 Common Merganser 1 Red-breasted Merganser 1 Ruddy Duck 1 Partridge, Grouse, Turkey Gray Partridge 1 Ring-necked Pheasant 1 Ruffed Grouse 1 Spruce Grouse Sharp-tailed Grouse Greater Prairie-Chicken Wild Turkey 1 Loons Red-throated Loon Pacific Loon Common Loon 1 Grebes Pied-billed -

Record of Slaty-Breasted Rail Rallus Striatus Breeding in Dehradun, India Pankaj Kumar & R
Record of Slaty-breasted Rail Rallus striatus breeding in Dehradun, India Pankaj Kumar & R. Suresh Kumar Kumar, P. & Kumar, R. S. 2009. Record of Slaty-breasted Rail Rallus striatus breeding in Dehradun, India. Indian Birds 5 (1): 21–22. Pankaj Kumar, Wildlife Institute of India, Post Box # 18, Chandrabani, Dehradun 248001, India. Email: [email protected] R. Suresh Kumar, Wildlife Institute of India, Post Box # 18, Chandrabani, Dehradun 248001, India. Email: [email protected] Mss received on 1st December 2008 he family Rallidae, represented by 19 spp. in India— WII campus is interspersed with a few perennial water sources including rails, crakes, gallinule, coot and finfoot—are small and a small man-made lake, and along these watercourses dense to medium-sized birds mainly inhabiting reedy swamps, stands of Sapium sebiferum grow and along some parts reeds of Tmangroves and paddy fields. A few of these species, specifically Typha elephantina occur, while the vegetation within the campus the rails and crakes, are notorious for their skulking habits and is marked by a luxuriant growth of sal Shorea robusta with a are rarely flushed, and are thus comparatively little known. One dense under-storey dominated by Lantana camara and Jasminum such species, the Slaty-breasted Rail Rallus striatus was observed in multiflorum bushes. the Wildlife Institute of India (WII) campus located in Dehradun. The rail was again seen at the same location on 1st August This species was also observed to breed inside the campus and 2007 and to our great surprise it was with eight precocious chicks! here we report on these observations.