Litigation Relevant to Regulation of Novel and Emerging Nicotine and Tobacco Products
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

European Electronic Cigarette & Vaporizer Market
EUROPEAN ELECTRONIC CIGARETTE & VAPORIZER MARKET Size, Share, Analysis & Forecast: 2015-2025 European Electronic Cigarette & E Vapor Market Size, Share, Analysis & Forecast: 2015-2025 BIS Research is a leading market intelligence and technology research company. BIS Research publishes in-depth market intelligence reports focusing on the market estimations, technology analysis, emerging high-growth applications, deeply segmented granular country-level market data and other important market parameters useful in the strategic decision making for senior management. BIS Research provides multi-client reports, company profiles, databases, and custom research services. Copyright © 2015 BIS Research All Rights Reserved. This document contains highly confidential information and is the sole property of BIS Research. Disclosing, copying, circulating, quoting or otherwise reproducing any or all contents of this document is strictly prohibited. Access to this information is provided exclusively for the benefit of the people or organization concerned. It may not be accessed by, or offered whether for sale or otherwise to any third party. BIS Research Sample Pages 2 European Electronic Cigarette & E Vapor Market Size, Share, Analysis & Forecast: 2015-2025 1 E-CIGARETTE MARKET DYNAMICS 1.1 INTRODUCTION The market dynamics section of the report examines the diverse factors which govern the production and consumption of e-cigarettes in the European market. This analysis will provide an in-depth understanding of the direction in which the market is headed and the impacts of various factors on it. This section covers the market dynamics – namely the drivers, challenges, and the opportunities in the electronic cigarette market, listing and analyzing several factors that positively and negatively affect it. -
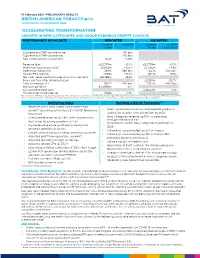
FY 2020 Announcement.Pdf
17 February 2021 –PRELIMINARY RESULTS BRITISH AMERICAN TOBACCO p.l.c. YEAR ENDED 31 DECEMBER 2020 ‘ACCELERATING TRANSFORMATION’ GROWTH IN NEW CATEGORIES AND GROUP EARNINGS DESPITE COVID-19 PERFORMANCE HIGHLIGHTS REPORTED ADJUSTED Current Vs 2019 Current Vs 2019 rates Rates (constant) Cigarette and THP volume share +30 bps Cigarette and THP value share +20 bps Non-Combustibles consumers1 13.5m +3.0m Revenue (£m) £25,776m -0.4% £25,776m +3.3% Profit from operations (£m) £9,962m +10.5% £11,365m +4.8% Operating margin (%) +38.6% +380 bps +44.1% +100 bps2 Diluted EPS (pence) 278.9p +12.0% 331.7p +5.5% Net cash generated from operating activities (£m) £9,786m +8.8% Free cash flow after dividends (£m) £2,550m +32.7% Cash conversion (%)2 98.2% -160 bps 103.0% +650 bps Borrowings3 (£m) £43,968m -3.1% Adjusted Net Debt (£m) £39,451m -5.3% Dividend per share (pence) 215.6p +2.5% The use of non-GAAP measures, including adjusting items and constant currencies, are further discussed on pages 48 to 53, with reconciliations from the most comparable IFRS measure provided. Note – 1. Internal estimate. 2. Movement in adjusted operating margin and operating cash conversion are provided at current rates. 3. Borrowings includes lease liabilities. Delivering today Building A Better TomorrowTM • Revenue, profit from operations and earnings • 1 growth* absorbing estimated 2.5% COVID-19 revenue 13.5m consumers of our non-combustible products , headwind adding 3m in 2020. On track to 50m by 2030 • New Categories revenue up 15%*, accelerating • Combustible revenue -

Spring 2003 Vol
ISSN 1536-4003 University of California, Berkeley Center forSlavic and East European Studies Newsletter Notes from the Director Spring 2003 Vol. 20, No. 1 In these unsettled times, it behooves us more than ever to understand the In this issue: causes and consequences of violence inflicted by extremist groups. The Notes from the Director .....................1 United States is not alone in facing profound challenges following the Bear Treks ........................................2 terrorist attacks of September 11, 2001. Like us, our West European allies are Berkeley-Stanford Conference ...........3 struggling to find a reasonable balance between respect for civil liberties Spring Courses .................................4 and freedom of expression on the one hand and the need to protect society Michael Carpenter from acts of terrorism carried out in the name of extremist ideologies. So, too, The Modern Slave Trade in is Russia wrestling with these issues, as highlighted most dramatically by Post-Communist Europe ...................5 the ongoing violence and human rights abuses being perpetrated by federal Michael Kunichika forces in Chechnya, as well as human rights violations and acts of terrorism A Window On the Lake: On and by the Chechen resistance, such as the recent hostage-taking incident in Around Stalin's Belomorkanal ...........9 Moscow. Nor should it be assumed that the only extremist challenge in Campus Visitors ............................. 16 Russia, or indeed elsewhere, comes from Islamist extremists. As Vladimir Christine Kulke Putin put it in his state of the nation address on April 18, 2002: “The growth Book Review: Historical Atlas of of extremism presents a serious threat to stability and public safety in our Central Europe ............................... -

Autumn 2007 Full Issue the .SU
Naval War College Review Volume 60 Article 1 Number 4 Autumn 2007 Autumn 2007 Full Issue The .SU . Naval War College Follow this and additional works at: https://digital-commons.usnwc.edu/nwc-review Recommended Citation Naval War College, The .SU . (2007) "Autumn 2007 Full Issue," Naval War College Review: Vol. 60 : No. 4 , Article 1. Available at: https://digital-commons.usnwc.edu/nwc-review/vol60/iss4/1 This Full Issue is brought to you for free and open access by the Journals at U.S. Naval War College Digital Commons. It has been accepted for inclusion in Naval War College Review by an authorized editor of U.S. Naval War College Digital Commons. For more information, please contact [email protected]. 1 Autumn 2007 60, Number 4 Volume Naval War College: Autumn 2007 Full Issue NAVAL WAR COLLEGE REVIEW Published by U.S. Naval War College Digital Commons, 2007 NAVAL WAR COLLEGE REVIEW Autumn 2007 R COL WA LEG L E A A I V R A N O T C I V I R A M S U S E B I T A T R T I H S E V D U E N T I Color profile: Disabled Composite Default screen Naval War College Review, Vol. 60 [2007], No. 4, Art. 1 Cover The Kongo-class guided-missile destroyer JDS Chokai (DDF 176) of the Japan Mar- itime Self-Defense Force alongside USS Kitty Hawk (CV 63) on 10 December 2002. The scene is evocative of one of the many levels at which the “thousand-ship navy,” examined in detail in this issue by Ronald E. -

Edinburgh Research Explorer
Edinburgh Research Explorer E-Cigarette Uptake and Marketing Citation for published version: Bauld, L, Angus, K & De Andrade, M 2014, E-Cigarette Uptake and Marketing: A Report Commissioned by Public Health England. Public Health England. <https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/311491/Ecigarette_uptake_ and_marketing.pdf> Link: Link to publication record in Edinburgh Research Explorer Document Version: Publisher's PDF, also known as Version of record Publisher Rights Statement: © Bauld, L., Angus, K., & De Andrade, M. (2014). E-Cigarette Uptake and Marketing: A Report Commissioned by Public Health England. Public Health England. General rights Copyright for the publications made accessible via the Edinburgh Research Explorer is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The University of Edinburgh has made every reasonable effort to ensure that Edinburgh Research Explorer content complies with UK legislation. If you believe that the public display of this file breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. Download date: 07. Oct. 2021 E-cigarette uptake and marketing! A report commissioned by Public Health England Authors: Professor Linda Bauld, Kathryn Angus and Dr Marisa de Andrade Institute for Social Marketing University of Stirling E-cigarette uptake and marketing About Public Health England Public Health England’s mission is to protect and improve the nation’s health and to address inequalities through working with national and local government, the NHS, industry and the voluntary and community sector. -

ASA Adjudication on Ten Motives Ltd
ASA Adjudication on Ten Motives Ltd Ten Motives Ltd Suite 15 Edwin Foden Business Centre Moss Lane Sandbach Cheshire CW11 3AE Date: 12 March 2014 Media: Brochure Sector: Leisure Number of complaints: 1 Complaint Ref: A13-250753 Background Summary of Council decision: Two issues were investigated, both of which were Upheld. Ad A leaflet for e-cigarettes, which featured the "Ten Motives disposable electronic cigarette" and pictured two versions of the product, stated "The healthier smoking alternative", and "... because it contains no tar or cancerous toxins, you can still enjoy smoking without worrying about the effects on your health". Issue The complainant challenged whether the following claims were misleading and could be substantiated: 1. "The healthier smoking alternative"; and, 2. "you can still enjoy smoking without worrying about the effects on your health". CAP Code (Edition 12) 3.13.7 Response Ten Motives Ltd stated that the MHRA had confirmed that it was acceptable to refer to their product as a healthier alternative. They stated that ASH were the recognised authority on smoking in the UK and quoted from their briefing document in June 2013, which stated "In 1976 Professor Michael Russell wrote: ‘People smoke for nicotine but they die from the tar’. Indeed, the harm from smoking is caused almost exclusively by toxins present in tobacco released through combustion. By contrast, pure nicotine products, although addictive, are considerably less harmful. Electronic cigarettes consequently represent a safer alternative to cigarettes for smokers who are unable or unwilling to stop using nicotine". They also provided links to the presentations given at an 'E-cig summit' in November 2013 at the Royal Society, which they stated had been attended by recognised international scientific authorities on smoking, including the MHRA. -

Litigation Relevant to Regulation of Novel and Emerging Nicotine and Tobacco Products
Case summaries LITIGATION RELEVANT TO REGULATION OF NOVEL AND EMERGING NICOTINE AND TOBACCO PRODUCTS COMPARISONCASE SUMMARIES ACROSS JURISDICTIONS Benn McGrady and Kritika Khanijo A Case summaries B Case summaries LITIGATION RELEVANT TO REGULATION OF NOVEL AND EMERGING NICOTINE AND TOBACCO PRODUCTS CASE SUMMARIES C Litigation relevant to regulation of novel and emerging nicotine and tobacco products: case summaries ISBN 978-92-4-002418-2 (electronic version) ISBN 978-92-4-002419-9 (print version) © World Health Organization 2021 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization (http://www.wipo.int/amc/en/mediation/rules/). -

Study on Council Directive 2011/64/EU on the Structure and Rates of Excise Duty Applied to Manufactured Tobacco
Study on Council Directive 2011/64/EU on the structure and rates of excise duty applied to manufactured tobacco Final Report Volume 1 – Main Text May 2017 EUROPEAN COMMISSION Directorate General for Taxation and Customs Union Directorate C – Indirect Taxation and Tax Administration Unit C.2 – Indirect Taxes other than VAT Contact: Annerie Bouw E-mail: [email protected] European Commission B-1049 Brussels 2 Europe Direct is a service to help you find answers to your questions about the European Union. Freephone number (*): 00 800 6 7 8 9 10 11 (*) The information given is free, as are most calls (though some operators, phone boxes or hotels may charge you). LEGAL NOTICE The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the Commission. The Commission does not guarantee the accuracy of the data included in this study. Neither the Commission nor any person acting on the Commission’s behalf may be held responsible for the use which may be made of the information contained therein. More information on the European Union is available on the Internet (http://www.europa.eu). Luxembourg: Publications Office of the European Union, 2017 ISBN 978-92-79-70215-0 doi: 10.2778/667238 © European Union, 2017 Reproduction is authorised provided the source is acknowledged. 3 Study on Council Directive 2011/64/EU on the structure and rates of excise duty applied to manufactured tobacco Authors: Tommaso Grassi (Team leader) Giacomo Luchetta Supported by: Maria Grandinson Carlo Montino Grzegorz Poniatowski 4 Study on Council Directive 2011/64/EU on the structure and rates of excise duty applied to manufactured tobacco Table of Contents Acronyms and Abbreviations.................................................................................. -

A Companion to Andrei Platonov's the Foundation
A Companion to Andrei Platonov’s The Foundation Pit Studies in Russian and Slavic Literatures, Cultures and History Series Editor: Lazar Fleishman A Companion to Andrei Platonov’s The Foundation Pit Thomas Seifrid University of Southern California Boston 2009 Copyright © 2009 Academic Studies Press All rights reserved ISBN 978-1-934843-57-4 Book design by Ivan Grave Published by Academic Studies Press in 2009 28 Montfern Avenue Brighton, MA 02135, USA [email protected] www.academicstudiespress.com iv Effective December 12th, 2017, this book will be subject to a CC-BY-NC license. To view a copy of this license, visit https://creativecommons.org/licenses/by-nc/4.0/. Other than as provided by these licenses, no part of this book may be reproduced, transmitted, or displayed by any electronic or mechanical means without permission from the publisher or as permitted by law. The open access publication of this volume is made possible by: This open access publication is part of a project supported by The Andrew W. Mellon Foundation Humanities Open Book initiative, which includes the open access release of several Academic Studies Press volumes. To view more titles available as free ebooks and to learn more about this project, please visit borderlinesfoundation.org/open. Published by Academic Studies Press 28 Montfern Avenue Brighton, MA 02135, USA [email protected] www.academicstudiespress.com CONTENTS CHAPTER ONE Platonov’s Life . 1 CHAPTER TWO Intellectual Influences on Platonov . 33 CHAPTER THREE The Literary Context of The Foundation Pit . 59 CHAPTER FOUR The Political Context of The Foundation Pit . 81 CHAPTER FIVE The Foundation Pit Itself . -

Of Zandera Limited
Anticipated acquisition by Emerging Products Holding BV (a wholly-owned subsidiary of Japan Tobacco Int) of Zandera Limited ME/6457/14 Summary 1. Japan Tobacco International (JTI) has agreed to acquire Zandera Limited, owner of the E-Lites e-cigarette brand (E-Lites) (the Merger). JTI is one of the UK’s largest suppliers of tobacco products, with no current e-cigarette offering. E-Lites currently supplies e-cigarettes but does not supply tobacco products. 2. The Competition and Markets Authority (CMA) gave notice to the parties that their Merger Notice was satisfactory on 14 July 2014. The CMA’s statutory timetable under section 34ZA(1) of the Enterprise Act 2002 (the Act) therefore expires on 8 September 2014. 3. As regards the product scope of the frame of reference, the CMA found that evidence from retailers and suppliers indicates that, although there is some degree of substitution between tobacco products and e-cigarettes, they are not currently close substitutes. As such, the CMA considers that they are likely to be in separate markets, although it was not necessary to conclude on this as no concerns arise on any basis. To the extent that there may be differences between the retail and online channels, the CMA has assessed the impact on them separately, while not concluding that they form distinct markets. 4. As regards geographic scope, the evidence received by the CMA indicates that it is likely to be at least as wide as national. The CMA has therefore assessed the impact of the Merger on the UK market. 5. -

WNTD 2020 Report-SEATCA-Final.Pdf
TODAY’S TEENS, TOMORROW’S CUSTOMERS: Baiting youths with new tobacco products to create a new generation of addicts Authors Worrawan Jirathanapiwat and Jennie Lyn Reyes Editorial team Southeast Asia Tobacco Control Alliance (SEATCA) Suggested citation Jirathanapiwat, W. and Reyes, J.L. (2020). Today’s teens, Tomorrow’s customers: Baiting youths with new tobacco products to create a new generation of addicts. Bangkok: Southeast Asia Tobacco Control Alliance. Cover Design Nichamon Pichiansathian Layout Watcharachai Ninthomya Published by Southeast Asia Tobacco Control Alliance (SEATCA) Thakolsuk Place, Room 4D, 115 Thoddamri Road, Dusit, Bangkok 10300 Thailand. Disclaimer The information, findings, interpretations, and conclusions expressed herein are those of the author(s) and do not necessarily reflect the views of the funding organization, its staff, or its Board of Directors. While reasonable efforts have been made to ensure the accuracy of information presented in this report at the time of publication, SEATCA does not warrant that the information in this document is complete and correct and shall not be liable for any damages incurred as a result of its use. Any factual errors or omissions are unintentional. For any corrections, please contact SEATCA via email: [email protected]. © Southeast Asia Tobacco Control Alliance 2020 This document is the intellectual property of SEATCA and its authors. SEATCA retains copyright on all text and graphic images in this document, unless indicated otherwise. This copyright is protected by domestic and copyright laws and international treaty provisions. The information in this document is made available for non-commercial use only. You may store the contents on your own computer or print copies of the information for your own non-commercial use. -

E-Cigarette Use Among Youth and Young Adults: a Report of the Surgeon General
E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General 2016 U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES Public Health Service Office of the Surgeon General Rockville, MD National Library of Medicine Cataloging-in-Publication Data Names: United States. Public Health Service. Office of the Surgeon General, issuing body. | National Center for Chronic Disease Prevention and Health Promotion (U.S.). Office on Smoking and Health, issuing body. Title: E-cigarette use among youth and young adults : a report of the Surgeon General. Description: Atlanta, GA : U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2016. | Includes bibliographical references. Subjects: MESH: Electronic Cigarettes – utilization. | Smoking – adverse effects. | Electronic Cigarettes – adverse effects. | Tobacco Industry. | Young Adult. | Adolescent. | United States. Classification: NLM QV 137 U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Chronic Disease Prevention and Health Promotion Office on Smoking and Health For more information For more information about the Surgeon General’s report, visit www.surgeongeneral.gov. To download copies of this document, go to www.cdc.gov/tobacco. To order copies of this document, go to www.cdc.gov/tobacco and click on Publications Catalog or call 1-800-CDC-INFO (1-800-232-4636); TTY: 1-888-232-6348. Suggested Citation U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S.