Behavioral Responses of the Endemic Shrimp
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Two Freshwater Shrimp Species of the Genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the Description of a New Species
A peer-reviewed open-access journal ZooKeys 923: 15–32 (2020) Caridina tetrazona 15 doi: 10.3897/zookeys.923.48593 RESEarcH articLE http://zookeys.pensoft.net Launched to accelerate biodiversity research Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species Qing-Hua Chen1, Wen-Jian Chen2, Xiao-Zhuang Zheng2, Zhao-Liang Guo2 1 South China Institute of Environmental Sciences, Ministry of Ecology and Environment, Guangzhou 510520, Guangdong Province, China 2 Department of Animal Science, School of Life Science and Enginee- ring, Foshan University, Foshan 528231, Guangdong Province, China Corresponding author: Zhao-Liang Guo ([email protected]) Academic editor: I.S. Wehrtmann | Received 19 November 2019 | Accepted 7 February 2020 | Published 1 April 2020 http://zoobank.org/138A88CC-DF41-437A-BA1A-CB93E3E36D62 Citation: Chen Q-H, Chen W-J, Zheng X-Z, Guo Z-L (2020) Two freshwater shrimp species of the genus Caridina (Decapoda, Caridea, Atyidae) from Dawanshan Island, Guangdong, China, with the description of a new species. ZooKeys 923: 15–32. https://doi.org/10.3897/zookeys.923.48593 Abstract A faunistic and ecological survey was conducted to document the diversity of freshwater atyid shrimps of Dawanshan Island. Two species of Caridina that occur on this island were documented and discussed. One of these, Caridina tetrazona sp. nov. is described and illustrated as new to science. It can be easily distinguished from its congeners based on a combination of characters, which includes a short rostrum, the shape of the endopod of the male first pleopod, the segmental ratios of antennular peduncle and third maxilliped, the slender scaphocerite, and the absence of a median projection on the posterior margin. -

Anchialine Cave Biology in the Era of Speleogenomics Jorge L
International Journal of Speleology 45 (2) 149-170 Tampa, FL (USA) May 2016 Available online at scholarcommons.usf.edu/ijs International Journal of Speleology Off icial Journal of Union Internationale de Spéléologie Life in the Underworld: Anchialine cave biology in the era of speleogenomics Jorge L. Pérez-Moreno1*, Thomas M. Iliffe2, and Heather D. Bracken-Grissom1 1Department of Biological Sciences, Florida International University, Biscayne Bay Campus, North Miami FL 33181, USA 2Department of Marine Biology, Texas A&M University at Galveston, Galveston, TX 77553, USA Abstract: Anchialine caves contain haline bodies of water with underground connections to the ocean and limited exposure to open air. Despite being found on islands and peninsular coastlines around the world, the isolation of anchialine systems has facilitated the evolution of high levels of endemism among their inhabitants. The unique characteristics of anchialine caves and of their predominantly crustacean biodiversity nominate them as particularly interesting study subjects for evolutionary biology. However, there is presently a distinct scarcity of modern molecular methods being employed in the study of anchialine cave ecosystems. The use of current and emerging molecular techniques, e.g., next-generation sequencing (NGS), bestows an exceptional opportunity to answer a variety of long-standing questions pertaining to the realms of speciation, biogeography, population genetics, and evolution, as well as the emergence of extraordinary morphological and physiological adaptations to these unique environments. The integration of NGS methodologies with traditional taxonomic and ecological methods will help elucidate the unique characteristics and evolutionary history of anchialine cave fauna, and thus the significance of their conservation in face of current and future anthropogenic threats. -
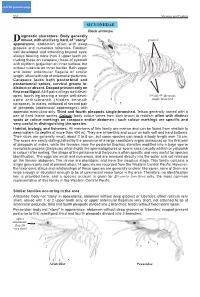
W7192e19.Pdf
click for previous page 952 Shrimps and Prawns Sicyoniidae SICYONIIDAE Rock shrimps iagnostic characters: Body generally Drobust, with shell very hard, of “stony” grooves appearance; abdomen often with deep grooves and numerous tubercles. Rostrum well developed and extending beyond eyes, always bearing more than 3 upper teeth (in- cluding those on carapace); base of eyestalk with styliform projection on inner surface, but without tubercle on inner border. Both upper and lower antennular flagella of similar length, attached to tip of antennular peduncle. 1 Carapace lacks both postorbital and postantennal spines, cervical groove in- distinct or absent. Exopod present only on first maxilliped. All 5 pairs of legs well devel- 2 oped, fourth leg bearing a single well-devel- 3rd and 4th pleopods 4 single-branched oped arthrobranch (hidden beneath 3 carapace). In males, endopod of second pair 5 of pleopods (abdominal appendages) with appendix masculina only. Third and fourth pleopods single-branched. Telson generally armed with a pair of fixed lateral spines. Colour: body colour varies from dark brown to reddish; often with distinct spots or colour markings on carapace and/or abdomen - such colour markings are specific and very useful in distinguishing the species. Habitat, biology, and fisheries: All members of this family are marine and can be found from shallow to deep waters (to depths of more than 400 m). They are all benthic and occur on both soft and hard bottoms. Their sizes are generally small, about 2 to 8 cm, but some species can reach a body length over 15 cm. The sexes are easily distinguished by the presence of a large copulatory organ (petasma) on the first pair of pleopods of males, while the females have the posterior thoracic sternites modified into a large sperm receptacle process (thelycum) which holds the spermatophores or sperm sacs (usually whitish or yellowish in colour) after mating. -

Perspectives on Typhlatya (Crustacea, Decapoda)
Contributions to Zoology, 65 (2) 79-99 (1995) SPB Academic Publishing bv, Amsterdam New perspectives on the evolution of the genus Typhlatya (Crustacea, Decapoda): first record of a cavernicolous atyid in the Iberian Peninsula, Typhlatya miravetensis n. sp. Sebastián Sanz & Dirk Platvoet 1 Unitat d'Ecologia, Facultat de Ciències Biologiques, Universitat de Valencia, E-46100 Burjassot, 2 Valencia, Spain; Institutefor Systematics and Population Biology (Zoological Museum, Amsterdam), University of Amsterdam, P.O. Box 94766, 1090 GT Amsterdam, The Netherlands Keywords: Typhlatya, Decapoda, Spain, subterranean waters, systematics, zoogeography, vicariance, evolution, key to genus Abstract historia geológica de la zona y la distribución mundial del género, del grupo de géneros, y la familia. On several occasions, shrimps belonging to a new species ofthe genus Typhlatya were collected in a cave in the province of Castellón, Spain. This is the first record of the in the genus Introduction Iberian Peninsula. The species is described and the validity, dis- tribution, and zoogeography of the genus, as well as the status In 1993 and were on several of the discussed. 1994, shrimps caught genus Spelaeocaris, are Former models for the occasions in in the evolution of the genus Typhlatya and its genus group are re- a cave near Cabanes, province viewed, as well asthe system ofinner classification of the Atyidae of Castellón, eastern Spain. The specimens belong and its For the and evolution of biogeographical meaning. age the to genus Typhlatya Creaser, 1936, a genus the genus we developed a new model based on vicariance prin- with members known from the Galápagos Islands, ciples that involves further evolution of each species after the Ascension and the Caribbean of the ancestral This allows estimations Island, Bermuda, disruption range. -

Behavioral Responses of the Endemic Shrimp Halocaridina Rubra
Behavioral Responses of the Endemic Shrimp Halocaridina rubra (Malacostraca: Atyidae) to an Introduced Fish, Gambusia affinis (Actinopterygii: Poeciliidae) and Implications for the Trophic Structure of Hawaiian Anchialine Ponds1 Krista A. Capps,2 Caroline B. Turner,2 Michael T. Booth,2 Danica L. Lombardozzi,2 Scott H. McArt,4 David Chai,3 and Nelson G. Hairston Jr.2,5 Abstract: In the Hawaiian Islands, intentionally introduced exotic fishes have been linked to changes in native biodiversity and community composition. In 1905, the mosquito fish Gambusia affinis was introduced to control mosquitoes. Subsequently, G. affinis spread throughout the Islands and into coastal anchia- line ponds. Previous studies suggest that presence of invasive fishes in anchialine ponds may eliminate native species, including the endemic shrimp Halocaridina rubra. We examined effects of G. affinis on H. rubra populations in anchialine ponds on the Kona-Kohala coast of the island of Hawai‘i. In the presence of G. affinis, H. rubra exhibited a diel activity pattern that was not seen in fishless ponds. Shrimp in ponds with fish were active only at night. This pattern was ev- ident in anchialine ponds and in laboratory experiments. In laboratory predation experiments, G. affinis preferentially consumed smaller H. rubra, and in the field the H. rubra collected from invaded sites were larger than those from fishless ponds. Analysis of trophic position using stable isotope analyses showed that feeding of H. rubra was not significantly distinct from that of snails, assumed to feed at trophic level 2.0 on epilithic algae, but G. affinis was slightly omnivo- rous, feeding at tropic level 2.2. -

Cave Biodiversity of the Southern Cumberland Plateau Kirk S
b-3-guidebook_Guidebook3 6/18/2014 10:01 PM Page 159 Cave Biodiversity of the Southern Cumberland Plateau Kirk S. Zigler, NSS 62696; Matthew L. Niemiller, NSS 53235; and Danté B. Fenolio The South Cumberland Region of Tennessee, Alabama, and Georgia (Figure 1) is known for its tremendous diversity of caves, including huge pits, massive stream passages, and tight crawls. Less well known is that the region also supports tremendous cave biodiversity (Niemiller, Zigler, and Fenolio, 2013). Here we discuss many of the species that inhabit caves of the region, focusing on the southern Cumberland Plateau. Cave Biodiversity Four ecological classes of organisms can be found in caves: trogloxenes, subtroglophiles, eutroglophiles, and troglobionts (Culver and Pipan, 2009). Trogloxenes are not typically found in caves and cannot persist there for long periods of time. They must either find their way back to the surface or ultimately perish. Subtroglophiles are commonly found in caves but are associated with surface habitats for at least part of their life cycle. Some are seasonal inhabitants of caves and others move back and forth from cave to surface habitats for feeding, such as cave-roosting bats, cave crickets, and Allegheny Woodrats (Neotoma magister). Eutroglophiles are commonly found underground but can be found in surface habitats. Unlike trogloxenes and subtroglophiles, eutroglophiles can complete their entire life cycle Figure 1 - The South Cumberland Region at the junction of underground. Examples include the Cave Salamander Tennessee, Alabama, and Georgia. Figure courtesy of Nick Hollingshead. (Eurycea lucifuga) and the Cave Orbweaver (Meta ovalis). Troglobionts are obligate, permanent residents of subterranean habitats. -

Index to Volume 53
Index to Volume 53 Author Index ABURTO-OROPEZA, OCTAVIo-see Sala et al. EWING, CURTIS ALLEN, MELINDA S., and GAIL M. MURAKAMI The Endemic Hawaiian Sap Beetles (Coleoptera: Liina'i Island's Arid Lowland Vegetation in Late Nitidulidae): Distribution, Ecological Shifts, and Prehistory, 88-112 Adaptation (abstract), 114-115 ARREOLA-RoBLES, JosE L.-see Sala et al. ASQUITH, ADAM-see Kido et al. ATAN, HUGO-see Osorio et al. FILARDI, CHRISTOPHER E., CATHERINE E. SMITH, ATKINSON, MARLIN J.-see Tarrant et al. ANDREW W. KRATTER, DAVID W. STEADMAN, and ATKINSON, SHANNON-See Tarrant et al. H. PRICE WEBB New Behavioral, Ecological, and Biogeographic Data BAILEy-BROCK, JULIE H. on the Avifauna of Rennell, Solomon Islands, 319 NeriIlidae of Hawai'i: Two New Records of 340 Interstitial Polychaetes, 299-304 FLETCHER, CHARLES H., III-see Harney et al. see also Brock et al. FOALE, SIMON BAILEy-BROCK, JULIE H., VERNON R. BROCK, and Local Ecological Knowledge and Biology of the Land RiCHARD E. BROCK Crab Cardisoma hirtipes (Decapoda: Gecarcinidae) Intrusion of Anchialine Species in the Marine at West Nggela, Solomon Islands, 37-49 Environment: The Appearance of an Endemic FOSTER, JAMIE S. Hawaiian Shrimp, Halocaridina rubra, on the South Lipopolysaccharide-Induced Apoptosis in the Shore ofO'ahu (Hawaiian Islands), 367-369 Symbiotic Light Organ of the Sepiolid Squid BIRKELAND, C. E.-see Green et al. Euprymna scolopes (abstract), liS BROCK, RICHARD E.-see Bailey-Brock et al. BROCK, RiCHARD, JULIE H. BAILEy-BROCK, and JOHN GARB, JESSICA E. GOODY Origins ofthe Hawaiian Crab Spider Fauna (abstract), A Case Study of Efficacy of Freshwater Immersion in 115-116 Controlling Introduction of Alien Marine Fouling GARRISON, JENNIFER Communities: The USS Missouri, 223-231 Role of Alien Tree Plantations in Native Forest BROCK, VERNON R.-see Bailey-Brock et al. -

On the Troglobitic Shrimps of the Yucatan Peninsula, Mexico (Decapoda: Atyidae and Palaemonidae)
On the Troglobitic Shrimps of the Yucatan Peninsula, Mexico (Decapoda: Atyidae and Palaemonidae) H. H. HOBBS III and HORTON H. HOBBS, JR. SMITHSONIAN CONTRIBUTIONS TO ZOOLOGY • NUMBER 240 SERIES PUBLICATIONS OF THE SMITHSONIAN INSTITUTION Emphasis upon publication as a means of "diffusing knowledge" was expressed by the first Secretary of the Smithsonian. In his formal plan for the Institution, Joseph Henry outlined a program that included the following statement: "It is proposed to publish a series of reports, giving an account of the new discoveries in science, and of the changes made from year to year in all branches of knowledge." This theme of basic research has been adhered to through the years by thousands of titles issued in series publications under the Smithsonian imprint, commencing with Smithsonian Contributions to Knowledge in 1848 and continuing with the following active series: Smithsonian Contributions to Anthropology Smithsonian Contributions to Astrophysics Smithsonian Contributions to Botany Smithsonian Contributions to the Earth Sciences Smithsonian Contributions to Paleobiology Smithsonian Contributions to Zoology Smithsonian Studies in Air and Space Smithsonian Studies in History and Technology In these series, the Institution publishes small papers and full-scale monographs that report the research and collections of its various museums and bureaux or of professional colleagues in the world cf science and scholarship. The publications are distributed by mailing lists to libraries, universities, and similar institutions throughout the world. Papers or monographs submitted for series publication are received by the Smithsonian Institution Press, subject to its own review for format and style, only through departments of the various Smithsonian museums or bureaux, where the manuscripts are given substantive review. -

Reproduction and Development in Halocaridina Rubra Holthuis, 1963 (Crustacea: Atyidae) Clarifies Larval Ecology in the Hawaiian Anchialine Ecosystem
Reference: Biol. Bull. 229: 134–142. (October 2015) © 2015 Marine Biological Laboratory Reproduction and Development in Halocaridina rubra Holthuis, 1963 (Crustacea: Atyidae) Clarifies Larval Ecology in the Hawaiian Anchialine Ecosystem JUSTIN C. HAVIRD*,†, REBECCA C. VAUGHT, DAVID A. WEESE‡, AND SCOTT R. SANTOS Department of Biological Sciences and Molette Laboratory for Climate Change and Environmental Studies, Auburn University, 101 Rouse Life Sciences Bldg., Auburn, Alabama 36849 Abstract. Larvae in aquatic habitats often develop in and significantly higher in brackish and seawater (88% and environments different from those they inhabit as adults. 72%, respectively). Correlated with this finding, identifi- Shrimp in the Atyidae exemplify this trend, as larvae of able gills capable of ion transport did not develop until many species require salt or brackish water for develop- metamorphosis into juveniles. Thus, early life stages of ment, while adults are freshwater-adapted. An exception H. rubra are apparently excluded from surface waters, within the Atyidae family is the “anchialine clade,” which which are characterized by lower and fluctuating salini- are euryhaline as adults and endemic to habitats with sub- ties. Instead, these stages are restricted to the subterra- terranean fresh and marine water influences. Although the nean (where there is higher and more stable salinity) Hawaiian anchialine atyid Halocaridina rubra is a strong portion of Hawaii’s anchialine habitats due to their in- osmoregulator, its larvae have never been observed in na- ability to tolerate low salinities. Taken together, these ture. Moreover, larval development in anchialine species is data contribute to the understudied area of larval ecology poorly studied. Here, reproductive trends in laboratory col- in the anchialine ecosystem. -

Anchialine Shrimps in the Waikolao Area Has Been Constant, Except for Increases in ‘Ōpae ‘Ula Abundance Since 1996
Anchialine Ponds Anchialine Pond Shrimps Antecaridina lauensis Calliasmata pholidota ‘Ōpae ‘ula or Halocaridina rubra Halocaridina palahemo Metabetaeus lohena Procaris hawaiana Palaemonella burnsi Vetericaris chaceoru Metabetaeus lohena Courtesy Karl Magnacca SPECIES STATUS: All Federally Listed as Candidates except Halocaridina All State Listed as Candidates except Halocaridina IUCN Red List - Not considered All Endemic except Antecaridina, Calliasmata, Metabeteus SPECIES INFORMATION: This group of species live in underground (hypogeal) environments and in anchialine ponds which have a mix of freshwater and seawater through underground connections to the sea. All of the species except A. lauensis, C. pholidota, and M. lohena are endemic to Hawaii. ‘Ōpae ‘ula reaches 1.5 centimeters (one-half inch) in length and is an herbivore that grazes on algal, bacterial, and diatom films growing on rocks and other hard substrates. They can also filter feed in mid-water and at the surface. The other species are all larger (up to five cm or two inches long) and some are predatory. M. lohena is a snapping shrimp and feeds on ‘ōpae ‘ula. C. pholidota feeds on crustaceans and polychaetes, while P. hawaiana has been seen feeding on shrimp. All have red color and reduced appendages. ‘Ōpae ‘ula carry about 12 fertilized eggs under their abdomen for a brood period of about 38 days. They reproduce one to two times per year. Lifespan of ‘ōpae ‘ula is long, up to 20 years in captivity. Less is known about the life history of the other species, but they are relatively long- lived for species in their taxa. A. lauensis and M. lohena can live six years. -

Disturbance in the Anchialine Ecosystem: Ramifications for Ecology and Physiology
Disturbance in the anchialine ecosystem: ramifications for ecology and physiology by Justin Chase Havird A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama May 3, 2014 Keywords: ecophysiology, invasive species, crustacean, osmoregulation, gene expression Copyright 2014 by Justin Chase Havird Approved by Scott R Santos, Chair, Associate Professor of Biological Sciences Raymond P. Henry, Professor of Biological Sciences Mark R. Liles, Associate Professor of Biological Sciences Alan E. Wilson, Associate Professor of Biological Sciences/Fisheries, Aquaculture, and Aquatic Sciences Abstract Habitats in the anchialine ecosystem are defined as coastal ponds, pools, and caves that lack surface connections to the open ocean, but possess both seawater and freshwater influences due to subterranean connections to the ocean and groundwater. Such habitats are rare worldwide, but are concentrated in the Hawaiian Islands. Organisms that live in these habitats must cope with changing salinities, variable oxygen regimes, high levels of UV radiation, and anthropogenic effects such as pollution and invasive species. Accordingly, such organisms represent an opportunity to shed light on environmental physiology and invasive species biology. However, few studies have investigated physiology or response to invasive species in anchialine organisms. Accordingly, the objective of this dissertation is to examine the effect of natural and anthropogenic disturbances on the physiology and ecology of anchialine organisms. Chapter 1 provides an introduction to the anchialine ecosystem and outlines the specific aims of the dissertation. Chapter 2 presents a series of field and laboratory based experiments investigating how endemic Hawaiian anchialine organisms have responded to invasive fishes. -

Hoffman Dissertation.Pdf
Determining the spatial and seasonal influences of microbial community composition and structure from the Hawaiian anchialine ecosystem by Stephanie Kimie Hoffman A dissertation submitted to the Graduate Faculty of Auburn University in partial fulfillment of the requirements for the Degree of Doctor of Philosophy Auburn, Alabama December 10, 2016 Keywords: anchialine, microbial ecology, Hawaii, laminated mat, spatial variation, seasonal variation Copyright 2016 by Stephanie Kimie Hoffman Approved by Scott R Santos, Chair, Professor of Biological Sciences Mark R Liles, Professor of Biological Sciences Todd D Steury, Associate Professor of Wildlife Sciences Robert S Boyd, Professor and Undergraduate Program Officer of Biological Sciences Abstract Characterized as coastal bodies of water lacking surface connections to the ocean but with subterranean connections to the ocean and groundwater, habitats belonging to the anchialine ecosystem occur worldwide in primarily tropical latitudes. Such habitats contain tidally fluctuating complex physical and chemical clines and great species richness and endemism. The Hawaiian Archipelago hosts the greatest concentration of anchialine habitats globally, and while the endemic atyid shrimp and keystone grazer Halocaridina rubra has been studied, little work has been conducted on the microbial communities forming the basis of this ecosystem’s food web. Thus, this dissertation seeks to fill the knowledge gap regarding the endemic microbial communities in the Hawaiian anchialine ecosystem, particularly regarding spatial and seasonal influences on community diversity, composition, and structure. Briefly, Chapter 1 introduces the anchialine ecosystem and specific aims of this dissertation. In Chapter 2, environmental factors driving diversity and spatial variation among Hawaiian anchialine microbial communities are explored. Specifically, each sampled habitat was influenced by a unique combination of environmental factors that correlated with correspondingly unique microbial communities.