Comparison of the Effectiveness of Intradermal Injection of Lidocaine, Iontophoretic Delivery of Lidocaine, and Topical Emla
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unit 4: Medication Administration Fundamental of Nursing
Unit 4: Medication Administration Fundamental of Nursing Unit 4: Medication Administration: Medication: Is a substance administered for the diagnosis, cure, treatment, relief, or prevention of disease. Six Rights of Medication Administration After paramedics have received the medication or fluid order, they should then administer the drug in question. In performing drug administration, pre-hospital care providers adhere to the six rights of medication administration: 1. Right patient 2. Right medication 3. Right dose 4. Right route 5. Right time 6. Right documentation Basic principle of nurse on drugs administration 1. The nurse must know the drug's prescribed dose, method of administration, actions, expected therapeutic effect, possible interactions with other drugs, and adverse effects. 2. The nurse must know the institution's administration procedures for the client's welfare and the nurse's legal protection. 3. The nurse must Review physician's order for completeness the client's name, date of the order, name of the drug, dose, rout, time of administration, and the physician's signature. 1 Unit 4: Medication Administration Fundamental of Nursing 4. The nurse discusses the medication and its actions with the client; recheck the medication order if the client disagrees with the dose or the physician's order. 5. The nurse must check the physician's order against the client's medication administration record for accuracy. 6. The nurse gives the patient the right to know about the medication he is receiving and the right to refuse it. Routes of Administration A: Enteral Tract Routes The common enteral routes of administration used in general medical practice are as follows: 1. -

Preparation of the Materials for an Injection �����������������������Ă���Ţ
PREPARATION OF THE MATERIALS FOR AN INJECTION Ovidiu Fabian, Emilia ăţ Learning objectives What you should know What is an injection What are the parenteral routes of administration of drugs; what types of injections are there What are the advantages of parenteral drug administration What is a syringe; which are the parts of the syringe and needle What information should be looked for in the prescription / medical indication What important information should be looked for on the syringe package What information should be sought on the package of the medicine to be administered What materials are necessary for the injection procedure The technique of dissolving medication and aspirating it into the syringe What is a container for sharp objects What you should do Choose the right materials for an injection Check the identity of the patient that is about to have the injection Check the ’medication Open the packaging for the syringe / needle Attach the needle to the syringe; loosen and remove the needle sheath Open a vial and aspire the contents into the syringe Dissolve a medicinal powder in the vial and absorb the contents into the syringe Place the filled syringe into a position that is convenient to use Disinfect the place of the future injection Keep material sterility throughout the entire procedure Discharge the needle into the container for sharp objects Definition Injection is a procedure by which a drug substance is introduced into tissues using a special needle and a syringe. Drug administration through injections -

Smallpox Vaccination by Intradermal Jet Injection* 1
Bull. Org. mond. San)e 1969, 41, 749-760 Bull. Wld Hlth Org. Smallpox Vaccination by Intradermal Jet Injection* 1. Introduction, Background and Results of Pilot Studies J. DONALD MILLAR,1 RONALD R. ROBERTO,2 HERTA WULFF,3 HERBERT A. WENNER4 & D. A. HENDERSON 5 Jet injection has met with great success in the rapid and effective mass administration of several immunizing agents. The recent development of a jet injector nozzle specifically designedfor intradermal inoculation suggested thepossible extension ofjet injector methodo- logy to mass smallpox vaccination. A total of 156 volunteer subjects, 16 unvaccinated and 140 vaccinated more than 5 years previously, received either undiluted smallpox vaccine by the multiple-pressure technique, or 0.1 ml of various dilutions of smallpox vaccine by jet injector using the new nozzle. Cutaneous and serological responses in revaccinees revealed that jet injection of diluted vaccine with a titre of 107 TCID50/ml was as effective as multiple-pressure inoculation of undiluted vaccine. Among the small number ofprimary vaccinees, jet injection ofdiluted vaccine with a titre of 106 TCID5o/ml appearedas effective as multiple-pressure inoculation of undiluted vaccine. No complications of vaccination occurred. The findings confirm the utility of the intradermal nozzle for jet injection of smallpox vaccine. In view of the speed ofadministration and the economy of vaccine, it is suggested that there is a distinct role for jet injection in global smallpox eradication efforts. Further studies on larger numbers of unvaccinated subjects and on persons with significant residual vaccinial immunity are needed to define the optimal concentration of vaccine for mass vaccination by iet injection. -

Injections in Dogs and Cats
SOP: Injections in Dogs and Cats These SOPs were developed by the Office of the University Veterinarian and reviewed by Virginia Tech IACUC to provide a reference and guidance to investigators during protocol preparation and IACUC reviewers during protocol review. They can be used as referenced descriptions for procedures on IACUC protocols. However, it is the sole responsibility of the Principal Investigator to ensure that the referenced SOPs adequately cover and accurately represent procedures to be undertaken in any research project. Any modification to procedure as described in the SOP must be outlined in each IACUC protocol application (e.g. if the Principal Investigator plans to use a needle size that is not referenced in the SOP, simply state that alteration in the IACUC protocol itself). Table of Contents I. Procedure Summary & Goal ......................................................................................... 1 II. Personal Protective Equipment & Hygiene ................................................................... 1 III. Supply List .................................................................................................................... 1 IV. Detailed Procedure ........................................................................................................ 1 V. Potential Adverse Effects, Mitigation, or Treatment .................................................... 2 VI. Variations ..................................................................................................................... -

Intradermal Therapy (Mesotherapy) in Derma- Tology
Canzona F, Mammucari M, Tuzi A, Maggiori E, Grosso MG, Antonaci L, Santini S, Catizzone AR, Troili F, Gallo A, Paolucci T, Rocchi P, Guglielmo C, Russo D, Giorgio C, Dorato D, Marzo RD, Viglione G, Fiorentini AG, Giardini M, Natoli S. Intradermal Therapy (mesotherapy) in Derma- tology. J Dermatol & Skin Sci. 2020;2(1):22-25 Mini-Review Article Open Access Intradermal Therapy (mesotherapy) in Dermatology Flora Canzona1, Mammucari Massimo2*, Arianna Tuzi3, Enrica Maggiori2, Maria Gabriella Grosso4, Luciano Antonaci2, Stefania Santini3, Anna Rosa Catizzone3, Fiammetta Troili3, Alessandra Gallo3, Teresa Paolucci3, Piergiovanni Rocchi3, Costanza Guglielmo3, Domenico Russo5, Chiara Giorgio6, Dario Dorato3, Raffaele Di Marzo3, Giovanna Viglione3, Anna G Fiorentini3, Manuela Giardini3, Silvia Natoli7. 1Istituto Dermopatico dell’Immacolata, IRCCS Foundation, Rome, Italy 2Primary Care Unit ASL RM 1, Rome, Italy 3Member of the Italian Society of Mesotherapy, Rome, Italy 4Ospedale Israelitico, Rome Italy 5San Marco Hospice and Palliative Care, Latina, Italy 6Rehabilitation Unit, F Pirinei Hospital, Altamura (BA), Italy 7Department of Clinical Science and Translational Medicine, Tor Vergata University, Rome, Italy Article Info Abstract Article Notes Mesotherapy consists of a series of micro injections in the superficial layer Received: January 28, 2020 of the skin of active ingredients that slowly diffuse into the underlying tissues. Accepted: March 17, 2020 This technique is applied in different clinical conditions and also in dermatology *Correspondence: it could play a useful role in the treatment path of many patients. However, *Dr.Mammucari Massimo, Primary Care Unit ASL RM 1, Rome, further clinical studies are needed to standardize its application in various Italy; Email: [email protected]. -
Intradermal Injection Simulator LF01008U Instruction Manual
Other Available Simulators LF00698U Adult Injectable Arm (Light) LF01162U Venatech IV Trainer LF00958U Pediatric Injectable Arm LF01184U Venatech IM & Sub Q LF00961U Intramuscular Injection LF00995U Arterial Puncture Arm LF01184U LF00999U Pediatric Injectable Head LF01005U First Aid Arm Intradermal Injection LF01037U Hemodialysis Practice Arm LF01121U Advanced IV Arm Simulator LF01131U Venipuncture and Injection Arm LF01139U Advanced IV Hand LF01008U Instruction Manual LF00961U LF01121U Fort Atkinson 901 Janesville Avenue, P.O. Box 901 Fort Atkinson, Wisconsin 53538-0901 1.800.558.9595 Products by Nasco eNasco.com • E-mail: [email protected] COPYRIGHT © NASCO 1982 PRINTED IN U.S.A. NP 53-82/RV 6-15 Other Available Simulators LF00698U Adult Injectable Arm (Light) LF01162U Venatech IV Trainer LF00958U Pediatric Injectable Arm LF01184U Venatech IM & Sub Q LF00961U Intramuscular Injection LF00995U Arterial Puncture Arm LF01184U LF00999U Pediatric Injectable Head LF01005U First Aid Arm Intradermal Injection LF01037U Hemodialysis Practice Arm LF01121U Advanced IV Arm Simulator LF01131U Venipuncture and Injection Arm LF01139U Advanced IV Hand LF01008U Instruction Manual LF00961U LF01121U Fort Atkinson 901 Janesville Avenue, P.O. Box 901 Fort Atkinson, Wisconsin 53538-0901 1.800.558.9595 Products by Nasco eNasco.com • E-mail: [email protected] COPYRIGHT © NASCO 1982 PRINTED IN U.S.A. NP 53-82/RV 6-15 NP053_82.indd 1 6/1/15 3:18 PM A. Care of the Intradermal Sites Puncturing the skin with needles forms small slits or cuts which will eventually lead to deterioration. Should leakage occur, perform the following steps: 1. Using the supplied intradermal sealant and supplied 22-gauge syringe, remove the needle end of the syringe by twisting at the luer lock. -

NEEDLE-FREE JET INJECTION BIBLIOGRAPHY, DEVICE & MANUFACTURER ROSTER, and PATENT LIST
JET INJECTION Bibliography p. 1 of 79 Bruce G. Weniger file: Jetinject-Bib.doc & .pdf last update: 2004-Apr-06 NEEDLE-FREE JET INJECTION BIBLIOGRAPHY, DEVICE & MANUFACTURER ROSTER, and PATENT LIST last update: 2004-April-06 (Available at: http://www.cdc.gov/nip/dev/jetinject.htm#bibliography) New or updated entries added since website-posting of 2003-Dec-24: References: Carter2003, Haupt2003 Devices: GunA, EMS/RPM, EMS/MPM Patents: US 6063053, 6080130, 6156008, 6223786, 6302160 B2, 6447475 B1, 6474369 B2, 6500239 B2, 6610042 B2, 6613010 B2, 6613011 B2 Acknowledgements: Bibliography initiation, 1994-1995 – Lisa Lindsay, Emory Univ. Russian translations/summaries, 1999-2002 – Dr. Vitali Pool, CDC German translation, 2003 – Dr. Katrin Kohl, CDC Bibliographic retrievals and collection, 1998-2000 – Robyn Kaiser, Emory Univ. Update, April-May, 2002 – Christine Korhonen, Emory Univ. Barclay1962 – Joanne Penkalski, Academy of Medicine Cleveland/Northern Ohio Medical Association Béclard/Galante1866, Galante1866/1885 – Bernadette Molitor & Marie Peyraube, Bibliothèque Interuniversitaire de Médecine, Paris, France Linder1979 – Darin Zehrung, PATH, Seattle, Washington Schön1965 – Urda Guse, Zentrale Hochschulbibliothek, Lübeck, Germany Spiegel1994a – Philippe Stoeckel, Association pour l’Aide à la Médecine Préventive, Paris; Betty Dodet, Fondation Mérieux, Lyon, France Legend: ~R.200#MonDay: Reprint copy requested on date indicated. Double-indented items: Paper copy not yet received or not yet filed. *ID: Article on intradermal vaccination but NOT via needle-free injection device. Kindly please address any additions, updates, corrections, and suggestions to: Bruce G. Weniger, MD bgw2(-at-)cdc.gov Fax: [+1] 404-639-8834 JET INJECTION Bibliography p. 2 of 79 Bruce G. Weniger file: Jetinject-Bib.doc & .pdf last update: 2004-Apr-06 REFERENCES {Abb1981} Abb J, Deinhardt F, Eisenberg J. -

Routes of Administration R UE of OUTES
C HAPTER32 Routes of Administration R OUTES OF Shinya Shimizu A DMINISTRATION National Institute of Animal Health,Tsukuba, Japan increases successful treatment. Personnel using experi- General mental animals should be well trained in handling and restraint, should obtain authentication for responsible Mice are the most widely used animals for a range of use of experimental animals and attain a scientifically experiments including medical, chemical, pharmaco- high standard (ETS 123, 1986; Nebendahl, 2000). 527 logical, toxicological, biological, and genetic. The Further experience will lead to repeatable and reliable administration of test substances, such as chemical ele- results (see Chapter 31 on Handling and Restraint). P ments, compounds, drugs, antibodies, cells or other During administration mice should be protected ROCEDURES agents, to mice is one of the major methods for evalu- from pain, suffering, distress or lasting harm or at least ating their biological activity. pain and distress shall be kept to a minimum (ETS The route of administration is largely dependent on 123, 1986). Some injections (such as footpad injec- the property of the test substance and the objective of tion) are strongly discouraged and if required must be the experiment. All administration should be performed justified on a case by case basis (CCAC, 2002). with knowledge of the chemical and physical characteris- tics of the substance. All routes have both demerit and merit, such as the absorption, bioavailability and metab- olism of the substance. Consideration should be paid to Principles of the pH, viscosity, concentration, sterility, pyrogenicity, toxicity as well as the existence of hazardous substances. administration A knowledge of available methods and techniques of administration as well as knowledge of the deposition Handling and restraint and fate of the administered substance will help the scien- tist/investigator to select the most appropriate route for Good handling and restraint is the most important her/his purpose. -
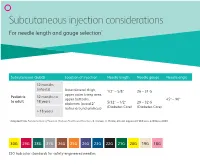
Subcutaneous Injection Considerations for Needle Length and Gauge Selection*
Subcutaneous injection considerations For needle length and gauge selection* Subcutaneous (SubQ) Location of injection Needle length Needle gauge Needle angle 12 months (infants) Anterolateral thigh, 1/2” – 5/8” 26 – 31 G upper outer tricep area, Pediatric 12 months to upper buttocks, 45º – 90º to adult 18 years abdomen (avoid 2” 5/32” – 1/2” 29 – 32 G radius around umilicus) (Diabetes Care) (Diabetes Care) > 18 years * Adapted from Fundamentals of Nursing: Human Health and Function. R. Craven, C. Hirnle, 4th ed. Lippincott Williams & Wilkins 2003. 30G 29G 28G 27G 26G 25G 24G 23G 22G 21G 20G 19G 18G ISO hub color standards for safety-engineered needles 50 45 40 35 30 25 Subcutaneous20 (SubQ) injection considerations: 15 10 4545° 9090° Injection procedure Outer aspect of the Outer aspect of the • Raise a fold of skin between upper thigh upper arm the thumb and forefinger, (not prefered site) and insert the needle at a 45º – 90º angle. • Pull back plunger slightly. If blood appears in syringe, remove needle, dispose properly and prepare a new injection.* * It is not necessary to aspirate for blood return when administering insulin or heparin. Abdomen—avoid Upper buttocks injecting within 2” around the umbilicus This information is being provided for convenience only and is not intended to replace clinical decision making. Each clinician is soley responsible for determinng the correct needle for each patient. bd.com BD and the BD Logo are trademarks of Becton, Dickinson and Company or its affiliates. All other trademarks are the property of their respective owners. © 2019 BD. All rights reserved. -

Nursing Procedure for Initiating Subcutaneous Fluid Administration with Hylenex Recombinant
Nursing Procedure for Initiating Subcutaneous Fluid Administration with hylenex recombinant PREP INSERT INFUSE Please see back cover for Indication and Important Safety Information and accompanying full Prescribing Information. These draft policies and procedures have been adapted from the Infusion Nurses Society (INS), the Oncology Nursing Society (ONS), and the hylenex recombinant (hyaluronidase human injection) prescribing information. It is the responsibility of the healthcare organization considering the use of this procedure to conduct an independent review and assessment to determine applicability and appropriateness of this document to their institution and to any particular clinical setting. Infusion therapies present risks; it is the responsibility of the healthcare organization to manage these risks, including the skills and competency validation of personnel. The healthcare organization that uses these guidelines should be aware that an annual review of organizational policies and procedures should continue to occur in accordance and compliance with regulatory and nonregulatory agencies. The Purpose of this Guide To outline nursing responsibilities in providing symptom control and/or fluid and electrolyte replacement by administering fluids subcutaneously. Policy Subcutaneous access is utilized to administer fluids via single injection or continuous infusion into the subcutaneous tissue. The fluid is absorbed through both adipose and connective tissue.1 hylenex recombinant is used for subcutaneous fluid administration, enabling enzymatically augmented subcutaneous infusion. In subcutaneous fluid administration, a catheter is placed in the subcutaneous tissue.1 Skilled and competent nurses knowledgeable in subcutaneous administration and the operation of electronic infusion devices may initiate and manage subcutaneous infusions.1 Some state and facility regulations allow this procedure to be performed by LVNs/LPNs. -

Brief Note on Inactive Ingredients Approved by USFDA
Brief note on Inactive Ingredients approved by USFDA FOR DIFFERENT DOSAGE FORMS WITH SPECIFIED ROUTE OF ADMINISTRATION Contents 1.0 Introduction………………..………………………………………………………………………………………………… 2.0 Inactive Ingredient Field Descriptions ….…………….....……………………………………………………… 3.0 Top Ten Inactive Ingredients……………….…………………………………………………………………………. a. List of Top 10 Inactive Ingredients (out of 3017) Approved by USFDA for specific dosage forms with specified route of administration-Pharmexcil Analysis (Table 1)….…………………….. 4.0 Top Ten Dosage Forms With Specific Route Of Administration And Number Of Inactive Ingredients……………………………………………………………………………………………………………………….. a. List indicating the number of inactive ingredients used in USFDA approved products for a specific route of administration and dosage form-Top 10-Pharmexcil Analysis (Table 2)…..….. b. Dosage form with specified route of administration vs. No. of Inactive ingredients approved by USFDA-Top 10- Pharmexcil Analysis (Figure 1)…...…………………………………………………………….. Figures: 1. Dosage form with specified route of administration vs. No. of Inactive ingredients approved by USFDA-Top 10- Pharmexcil Analysis Tables: 1. List of Top 10 Inactive Ingredients (out of 3017) Approved by USFDA for specific dosage forms with specified route of administration-Pharmexcil Analysis ………………………………..………………….. 2. List indicating the number of inactive ingredients used in USFDA approved products for a specific route of administration and dosage form-Top 10-Pharmexcil Analysis ……………..…..….. 3. List of Inactive ingredients approved by USFDA for specific dosage forms and route of administration – Pharmexcil Analysis………………………………………………………………………………………. 4. List indicating the number of inactive ingredients used in USFDA approved products for a specific route administration and dosage form – Pharmexcil Analysis ….………………………………….. 5. List of inactive ingredients for USFDA approved Drug Products………………………………………………... DISCLAIMER 1. The data should not be copied or transmitted to others in any means. -

Administration Routes
Administration Routes Mice & Rats -Intramuscular Injection (IM) IM injections are administered in the thigh muscles of the hind limb. Caution must be used to avoid injury to the sciatic nerve, which is located in close proximity to the femur. 1. Restrain the animal by grasping the skin along its back with your left hand (if right-handed). 2. Clean the injection site with alcohol. 3. Insert needle into thigh muscles, and directed away from the femur avoiding the sciatic nerve. 4. Pull back the syringe plunger to aspirate the syringe. Any blood indicates improper needle placement, and needle must be repositioned. 5. Administer substance in a steady, fluid motion. Take care not to administer fluid too rapidly. 2 Administration Routes Mice & Rats –Subcutaneous Injection (SQ or SC) SQ injections are administered in the space between the skin and the underlying muscles. 1. Restrain the animal by grasping the skin along its back with your left hand (if right-handed), as shown in picture. 2. Clean injection site with alcohol. 3. Insert needle at base of skin fold between thumb and finger. 4. Pull back the syringe plunger to aspirate the syringe. Any blood indicates improper needle placement, and needle must be repositioned. 5. Administer substance in a steady, fluid motion. 3 Administration Routes Mice & Rats -Intradermal Injection (ID) ID injections are administered between the layers of the skin. The animal must be under general anesthesia for this procedure. 1. Anesthetize animal as described in your approved Animal Study Proposal. 2. Clip hair on animal’s back. 3. Clean the injection site with alcohol.