Isolation and Characterization of an Alkaloid-Blocked Mutant of Claviceps Paspali
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Risk Assessment of Argyreia Nervosa
Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos Risk assessment of Argyreia nervosa RIVM letter report 2019-0210 W. Chen | L. de Wit-Bos RIVM letter report 2019-0210 Colophon © RIVM 2020 Parts of this publication may be reproduced, provided acknowledgement is given to the: National Institute for Public Health and the Environment, and the title and year of publication are cited. DOI 10.21945/RIVM-2019-0210 W. Chen (author), RIVM L. de Wit-Bos (author), RIVM Contact: Lianne de Wit Department of Food Safety (VVH) [email protected] This investigation was performed by order of NVWA, within the framework of 9.4.46 Published by: National Institute for Public Health and the Environment, RIVM P.O. Box1 | 3720 BA Bilthoven The Netherlands www.rivm.nl/en Page 2 of 42 RIVM letter report 2019-0210 Synopsis Risk assessment of Argyreia nervosa In the Netherlands, seeds from the plant Hawaiian Baby Woodrose (Argyreia nervosa) are being sold as a so-called ‘legal high’ in smart shops and by internet retailers. The use of these seeds is unsafe. They can cause hallucinogenic effects, nausea, vomiting, elevated heart rate, elevated blood pressure, (severe) fatigue and lethargy. These health effects can occur even when the seeds are consumed at the recommended dose. This is the conclusion of a risk assessment performed by RIVM. Hawaiian Baby Woodrose seeds are sold as raw seeds or in capsules. The raw seeds can be eaten as such, or after being crushed and dissolved in liquid (generally hot water). -

Phoretic Analysis of Ergot Alkaloids. Relations Mobility in the Cle Vine
Acta Pharm, Suecica 2, 357 (1965) Thin-layer chromatographic and thin-layer electro- phoretic analysis of ergot alkaloids.Relations between structure, RM value and electrophoretic mobility in the cle vine series STIG AGUREll DepartMent of PharmacOgnosy, Kunql, Farmaceuliska Insiitutei, StockhOLM, Sweden SUMMARY A thin-layer chromatographic and electrophoretic study of the ergot alkaloids has been made, to find rapid methods for the separation and identification of the known ergot alkaloids. The mobilities of ergot alkaloids in several useful chromatographic and electrophore- tic systems are recorded. Relations have been observed between structure and R" value in methanol-chloroform on Silica Gel G. A simple, rapid thin-layer electrophoretic technique has been de- vised for separation of ergot alkaloids, and a relation between structure and electrophoretic mobility is evident. Two-dimensional combinations of thin-layer chromatography and thin-layer electro- phoresis and chromatography are described. Numerous paper chromatographic procedures have been published for separation of the ergot alkaloids and their derivatives. Hofmann (1) and Genest & Farmilio (2) have recently listed these systems. The general advantages of thin-layer chromatography (TLC) over paper partition chromatography are well known: shorter time of equilibration and devel- opment, generally better resolution, smaller amounts of substance rc- quired, and wider choice of reagents. Several reports of TLC of ergot alkaloids have been published. In gene- ral, these investigations (2-6 and others) have dealt 'with limited groups of alkaloids, or with a specific problem involving at most a dozen of the 40 now known naturally occurring ergot alkaloids. Some paper chromate- .357 graphic systems using Iorrnamide-treated papers have also been adopted for thin-layer chromatographic use (7, 8). -

Electronic Supplementary Material (ESI) for RSC Advances. This Journal Is © the Royal Society of Chemistry 2017
Electronic Supplementary Material (ESI) for RSC Advances. This journal is © The Royal Society of Chemistry 2017 The physicochemical properties and NMR spectra of some ergot alkaloids are summarized as following. Especially, under the influence of acids, alkaloids or light, the carbo at the position 8 of D-lysergic acid derivatives can occur isomerization which 8R is changed into 8S to form the responding isomers, lead to the formation of α-ergotaminine, ergocryptinine, ergocorninine and ergocristinine which the NMR data are shown in Table S1, S2 and S3. Clavine Agroclavine: crystal (acetone), mp 198~203℃, soluble in benzene, ethanol, slightly soluble 1 in water. Molecular formula: C16H18N2, MW m/z: 238. The data of H-NMR (Pyridine-d5,220 13 MHz) and C-NMR (Pyridine-d5,15.08 MHz) {Bach, 1974 #48}are shown in Table S1 and S2. Elymoclavine: mp 250~252℃, Molecular formula: C16H18N2O, MW m/z: 254. The data of 1 13 H-NMR (CDCl3, 220 MHz) and C-NMR (CDCl3, 15.08 MHz){Bach, 1974 #48} of its acetate derivatives are shown in Table S1 and S2. 1 Festuclavine: mp 241℃, Molecular formula: C16H18N2, MW m/z: 238. The data of H-NMR 13 (CDCl3, 220 MHz) and C-NMR (CDCl3, 15.08 MHz) {Bach, 1974 #48}are shown in Table S1 and S2. Fumigaclavine B: mp 265~267℃, Molecular formula: C16H20N2O, MW m/z: 256. The data of 1 13 H-NMR (pyridine-d5,220 MHz) and C-NMR (pyridine-d5,15.08 MHz) {Bach, 1974 #48}are shown in Table S1 and S2. Ergoamides Ergometrine: white crystal (acetone), mp 162℃. -

Ergometrine Maleate
The European Agency for the Evaluation of Medicinal Products Veterinary Medicines Evaluation Unit EMEA/MRL/237/97-FINAL June 1999 COMMITTEE FOR VETERINARY MEDICINAL PRODUCTS ERGOMETRINE MALEATE SUMMARY REPORT 1. Ergometrine is a naturally occurring alkaloid found in ergot (Claviceps purpurea). It is classified as a water-soluble lysergic acid derivative, and is an orally-active stimulant of uterine contractions. The maleate salt (ergometrine maleate) exhibits greater stability than the free base and is the usual form in which the alkaloid is used in medicinal products. It is used in veterinary medicine in the control of postpartum uterine haemorrhage, removal of fluid from atonic uteri, to prevent pro-lapsed uteri, and judiciously in terms of timing to aid in suturing the uterus after caesarean section or in replacing an everted uterus. Dose regimens are: cows and mares: 2 to 5 mg/animal (intravenously or intramuscularly); ewes, goats and sows: 0.5 to 1 mg/animal (intramuscularly). In human medicine, it is used orally and parenterally in the prevention and treatment of postpartum haemorrhage caused by uterine atony and for the stimulation of uterine involution. Usual oral doses are 500 µg 3 times daily up to 1.8 mg daily (approximately 0.03 mg/kg bw). Ergot alkaloids have been reported to be present in flour from rye, wheat and barley in amounts ranging from 0.01 to 2.36 mg/kg flour. EU legislation restricts the maximum percentage of ergot tolerated in flour to 0.1%. Total daily human intake of ergot alkaloids from contaminated foodstuffs of plant origin has been estimated as up to 7.8 µg/person. -

Eurofins IAC Ergot Alkaloids 3Ml Product Code: EFLS3275 Tecna Code:IA768
Instruction of the Clean-up Process Using Eurofins IAC Ergot Alkaloids 3ml Product Code: EFLS3275 Tecna code:IA768 THE PRESENT DOCUMENT IS A FAC-SIMILE VERSION. When performing the assay, please use the kit insert. Clean-up of Commodity Extracts of Food and Feed Samples containing 12 Priority Ergot Alkaloids Via Immunoaffinity Chromatography and Subsequent HPLC Analysis with Fluorescence Detection 1. Target of 12 priority Ergot Alkaloids: The most abundant ergot alkaloides produced by fungus Claviceps purpurea comprise six pairs of epimeric ergot alkaloides which can be classified as priority ergot alkaloides1. They form the target of Eurofins IAC Ergot Alkaloids 3ml column. C8-(R)-Epimers (toxic) C8-(S)-Epimers (not toxic) Ergometrine Ergometrinine Ergotamine Ergotaminine Ergosine Ergosinine Ergocornine Ergocorninine Ergocryptine Ergocryptinine Ergocristine Ergocristinine Because both epimers can change to each other under certain conditions, e.g. under acidic or basic influence, in nature and/or during sampling/extraction/analysis, the non-toxic C8-(S)-epimers have to be included in determination as well. 2. Principle: This instruction of priority ergot alkaloid determination in food and feed focuses on the enrichment step of extract using immunoaffinity column (IAC) and quantification with HPLC. Accepted laboratory extraction and IAC treatment methods could essentially be maintained. Full performance of the IAC column is given if pronounced criteria of organic solvent tolerance, elution process and working range of Eurofins IAC Ergot Alkaloids 3ml column is followed. Many pretreatment methods of ergot alkaloids determination in food and feed show low sensitivity because of interfering substances if problematic matrices are applied. This method of content determination of ergot alkaloids combines the high selectivity of an immunoaffinity column (IAC) with its potential to concentrate analytes and additional step of purification by HPLC column. -

A COMPARISON Oit SOME UI TEE Being
A COMPARISON OIt SOME UI TEE ERGOT ALKALOIDS. being a Thesis Presented for the Degree of Doctor of Medicine Edinburgh University by Adam Cairns White, ßß.B., Ch.B., Ph.D., Wellcome Physiological Research Laboratories, Langley Court, Beckenham, Kent. INS, September, 1939. Com-parison alLome FrEot Alkaloids. A- Section I. Contents. Introduction pp 1-8 General Toxic Symptoms in:- Monkey pp. 8 - 27 Fowl pp. 27 - 34 Canary pp. 34 - 36 Ct pp. 36a - 43 Rabbit pp. 43 - 44 Guinea Pig pp. 44 - 45 Mice pp. 45 - 55 Frog p) 55 Discussion of Toxic Symptoms pp. 55 - 60 Actions on Temperature Regulation in:- Mice pp. 60 - 76 Rabbit pp. 77 - 96 -1- The ergot alkaloids, other than those that are molecular compounds, fail into two groups, a laevorotatory physiologically active group, and a dextrorotatory group which is considerably less active. Smith and Timmis drew attention to this fact in their paper on isoergine and isolysergic acids (1). In Table l I have assembled the ergot alkaloids in descending order of the number of constituent carbon atoms, and given, where possible, their rotations in chloroform. The figures are obtained from the papers of Smith and Timmis (1) (2). The decomposition products, ergine and isoergine have also been included. -2- 200 Table 1. (« } 5461 Ergotoxine C35114105N5 -226e Ergotinine 035H4106N5 +446° 191 Ergotinine C35H4106N5 +513° Ergotamine C33H3505N5 -1810 Ergotaminine C33113505N5 +4500 Ergosine C30113705N5 -1930 Ergosinine C30H3705N5 +5220 Ergotnetrine C19x2302N3 - Ergometrinine C19i-12302N3 . +5200 isoErgine C16H170 N3 Ergine C10170 N3 +598° -3- Since the publications of Smith and Timis (1) (2), Stoll and Burckhardt (3) have reported the isolation of yet another large moleculed pair of alkaloids, ergocristine and ergocristinine C35H3905N5 (this is the same gross formula as they give for ergotoxine) for which they have rotations (el )p in chloroform of -186° and +365e respectively. -

Clavine and Lysergic Acid Alkaloids in Varieties of Morning Glory .Pdf
Phytochcmlatry,1963. Vol. 2, pp. 65 to 70. PtrpamonPress Ltd. Printed in England CLAVINE AND LYSERGIC ACID ALKALOIDS IN VARIETIES OF MORNING GLORY* W. A. TABERand L. C. VINING Prairie Regional Laboratory. National Research Council of Canada. Saskatoon. Saskatchewan and R. A. HEAC~CR Psychiatric Research Unit, University Hospital, Saskatoon, Saskatchewan (Received 16 Judy 1962) Abstract-The seeds of a number of commercially available varieties of Morning Glory (fpomoeu and Convolvulus sp.) habe been found to contain clavine and lysergic acid alkaloids. Using thin layer and paper chromatographic methods, ergine. isoergine, ergometrine, ergometrinine, elymoclavine. penniclavine, and chanoclavine have been tentatively identified. Not all varieties of seed tested contained these alkaloids: one which contained a substantial amount also contained alkaloid in the leaves and stem of the mature plant. INTRODUCTION “OLOLIUQUI” and “Badoh Negro”, the seeds of the convolvulaceous Rivea corymbosa (L.) Hall. f. and Zpomoea violacea (L.), respectively, have long been used by the Aztec Indians to attain a state of mind suitable for divination during traditional religious ceremonies.‘.2 The active principle has only recently been isolated by Hofmann and co-workers; lysergic acid amide (isoergine), a known psychotomimetic, is present and is accompanied in the seed by ergine, elymoclavine, charm&vine and lysergol as well as other related substances in smaller amounts.8*4ss Subsequent studies in our laboratorie&’ showed that the alkaloids are present in the microbially sterile embryo, and also in the leaf and stem, but not the root, of the mature plant. From this evidence it was considered reasonably certain that the alkaloids are a true metabolic product of the plant and not of an invading microbial parasite or contaminant. -

7. Ergot Alkaloids and Other Metabolites of the Genus Claviceps
7. ERGOT ALKALOIDS AND OTHER METABOLITES OF THE GENUS CLAVICEPS MARTIN BUCHTA and LADISLAV CVAK Galena a.s. Opava, 747 70 Czech Republic 7.1. INTRODUCTION Both parasitic and saprophytic ergot is a producer of vast number of compounds from which alkaloids represent the most important group. Over 80 ergot alkaloids (EA) have as yet been isolated from diverse natural material, mainly from Claviceps strains, but also from other fungi and higher plants. Besides alkaloids and some other secondary metabolites (pigments and mycotoxins), ergot produces also some unusual primary metabolites of the lipidic and sacharidic nature. 7.2. ERGOT ALKALOIDS Only alkaloids isolated from different Claviceps species are described in this chapter. EA produced by other fungi and higher plants are the subject of the Chapter 18. Beside this limitation, nearly 70 ergot alkaloids, produced by genus Claviceps, are discussed here. 7.2.1. Ergot Alkaloid Structure All the EA are biosynthetically derived from L-tryptophane, that means indole is the base of their structure. Although the most of EA contains a tetra-cyclic ergoline system (Figure 1), there are some exceptions whose structure is created only by two or three cycles. Natural alkaloids can be divided into three groups according to their structure: The first, structurally simplest group, contains alkaloids of clavine type, the second group are simple amides of lysergic and paspalic acids and the third, most complex group is created by the alkaloids of peptidic type. Biosynthetically, the peptidic EA can be understand as tetrapeptides containing lysergic acid as the first member of the peptidic chain. The other amino acids of the tetrapeptide are variable, which provides the basis for a great diversity of this group of EA. -

Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method
toxins Article Cleaving Ergot Alkaloids by Hydrazinolysis—A Promising Approach for a Sum Parameter Screening Method Maximilian Kuner 1, Susanne Kühn 2, Hajo Haase 3 , Klas Meyer 1 and Matthias Koch 1,* 1 Bundesanstalt für Materialforschung und-prüfung (BAM), 12205 Berlin, Germany; [email protected] (M.K.); [email protected] (K.M.) 2 Institut Kirchhoff Berlin GmbH, 13347 Berlin, Germany; [email protected] 3 Department of Food Chemistry and Toxicology, Technische Universität Berlin, 10623 Berlin, Germany; [email protected] * Correspondence: [email protected] Abstract: Ergot alkaloids are mycotoxins formed by fungi of the Claviceps genus, which are some of the most common contaminants of food and feed worldwide. These toxins are a structurally heterogeneous group of compounds, sharing an ergoline backbone. Six structures and their cor- responding stereoisomers are typically quantified by either HPLC-FLD or HPLC-MS/MS and the values subsequently summed up to determine the total ergot alkaloid content. For the development of a screening method targeting all ergot alkaloids simultaneously, the alkaloids need to be transferred to one homogeneous structure: a lysergic acid derivative. In this study, two promising cleaving methods—acidic esterification and hydrazinolysis—are compared, using dihydroergocristine as a model compound. While the acidic esterification proved to be unsuitable, due to long reaction times and oxidation sensitivity, hydrazinolysis reached a quantitative yield in 40-60 min. Parallel workup of several samples is possible. An increasing effect on the reaction rate by the addition of ammonium iodide was demonstrated. Application of hydrazinolysis to a major ergot alkaloid mix Citation: Kuner, M.; Kühn, S.; Haase, solution showed that all ergopeptines were cleaved, but ergometrine/-inine was barely affected. -
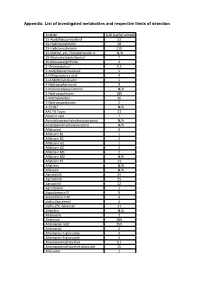
List of Investigated Metabolites and Respective Limits of Detection
Appendix: List of investigated metabolites and respective limits of detection Analyte LOD (µg/kg sample) 15-Acetyldeoxynivalenol 53 15-Hydroxyculmorin 18 15-Hydroxyculmoron 110 15-Methyl_epi_Fumiquinazolin A N/A 15-Monoacetoxyscirpenol 7 16-Ketoaspergillimide 2 2-Chlorunguinol 0,3 3-Acetyldeoxynivalenol 5 3-Nitropropionic acid 4 3-O-Methylvirdicatin 1 4-Hydroxyalternariol 9 4-Monoacetoxyscirpenol N/A 5-Hydroxyculmorin 180 5-Methylmellein 26 7-Hydroxypestalotin 2 A 23187 N/A AAL TA Toxin 21 Abscisic acid 7 Acetyldeoxypentahydroxyscirpenol N/A Acetylpentahydroxyscirpenol N/A Aflatoxicol 9 Aflatoxin B1 1 Aflatoxin B2 2 Aflatoxin G1 1 Aflatoxin G2 4 Aflatoxin M1 2 Aflatoxin M2 N/A Aflatoxin P1 11 Aflatrem N/A Aflavarin N/A Agistatin B 35 Agistatin D 25 Agistatin E 12 Agroclavine 1 Aigualomycin D 9 Alamethicin F30 4 alpha-Zearalenol 4 alpha-ZOL-Glucosid 11 Alteichin N/A Altenuene 2 Altenusin 280 Alternarian acid 350 Alternariol 2 Alternariol-3-glucoside 4 Alternariol-9-glucoside 4 Alternariolmethylether 0,1 Alternariolmethylether-glucoside 35 Altersetin 2 Altersolanol 110 Altertoxin-I 4 Altertoxin II N/A Amidepsin B 5 Aminooctadecyldecan-3-ol 21 Amoxycillin N/A Amphotericin N/A Anacin 28 Andrastin A 1 Andrastin B 2 Andrastin C N/A Andrastin D N/A Anisomycin 1 Anomalin A N/A Antibiotic F 1849 A 4 Antibiotic L 696474 71 Antibiotic PF 1052 7 Apicidin 1 Ascochlorin 1 Ascomycin 11 Asparason A N/A Aspercolorin 4 Asperfuran 71 Aspergamide A 9 Aspergillicin Derivat 4 Aspergillin PZ N/A Aspergillimide 1 Asperglauccide 0,4 Asperlactone 0,1 Asperloxin -

Validation of Ergot Alkaloids in Feeds by LC-MS/MS AAFCO Annual Meeting 2018 Mélanie Titley & Lise-Anne Prescott Ottawa Laboratory (Carling)
Validation of Ergot Alkaloids in Feeds by LC-MS/MS AAFCO Annual Meeting 2018 Mélanie Titley & Lise-Anne Prescott Ottawa Laboratory (Carling) © 2017 Her Majesty the Queen in Right of Canada (Canadian Food Inspection Agency), all rights reserved. Use without permission is prohibited. Overview • CFIA - OLC - FFC • What are Ergot Alkaloids (EA)? • Upcoming Regulations • Method Summary • Validation Results • Challenge - Standards • Next Steps CFIA - OLC - FFC • Regulatory analytical testing for CFIA enforcement and monitoring programs • Accredited for method development, validation and evaluation to meet CFIA’s changing needs • Expert scientific advice to Program officials, industry, other laboratories, national and international regulatory and scientific organizations What are Ergot Alkaloids? • Ergot Infection • Rye, triticale, wheat, barley, oats and grasses • Ergotism • What causes ergotism • Effect on humans • Effect on animals Dominique Jacquin, https://en.wikipedia.org/wiki/Ergotis m What are EAs? • The most common ergot alkaloids produced by Claviceps purpurea • Ergocornine, Ergocorninine • Ergocristine, Ergocristinine • Ergocryptine, Ergocryptinine • Ergometrine, Ergometrinine • Ergosine, Ergosinine • Ergotamine, Ergotamine Ergometrine (www.chemspider.com) • Epimers r-isomers (-ine) are biologically active • Epimers s-isomers (-inine) are less active Upcoming Regulations Proposed and current maximum levels for total ergot alkaloids Proposed Maximum Limit: Proposed Maximum Current Action Single Ingredient Limit: Level: Feeds (e.g., -

Effects of Continuously Feeding Diets Containing Cereal Ergot Alkaloids on Nutrient Digestibility, Alkaloid Recovery in Feces, and Performance Traits of Ram Lambs
toxins Article Effects of Continuously Feeding Diets Containing Cereal Ergot Alkaloids on Nutrient Digestibility, Alkaloid Recovery in Feces, and Performance Traits of Ram Lambs Stephanie Coufal-Majewski 1, Kim Stanford 2,* ID , Tim McAllister 3, Yuxi Wang 3, Barry Blakley 4, John McKinnon 5, Mary Lou Swift 6 and Alexandre V. Chaves 1 ID 1 Faculty of Science, School of Life and Environmental Sciences, University of Sydney, Sydney, NSW 2006, Australia; [email protected] (S.C.-M.); [email protected] (A.V.C.) 2 Livestock Research Section, Alberta Agriculture and Forestry, Lethbridge, AB T1J 4V6, Canada 3 Science and Technology Branch, Agriculture and Agri-Food Canada, Lethbridge, AB T1J 4B1, Canada; [email protected] (T.M.); [email protected] (Y.W.) 4 Department of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada; [email protected] 5 Department of Animal Science, University of Saskatchewan, Saskatoon, SK S7N 5B4, Canada; [email protected] 6 Ruminant Nutrition, Hi-Pro Feeds, Okotoks, AB T1S 1A2, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-403-381-5150 Received: 26 October 2017; Accepted: 14 December 2017; Published: 19 December 2017 Abstract: Allowable limits for cereal ergot alkaloids in livestock feeds are being re-examined, and the objective of this study was to compare nutrient digestibility, growth performance and carcass characteristics of ram lambs fed a range of alkaloid concentrations, including the maximum currently allowed in Canada (2 to 3 ppm). Four pelleted diets were fed: control, with no added alkaloids; 930; 1402; and 2447 ppb alkaloids based on total R and S epimers.