<I>Diopatra Cuprea</I>
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary
July 15, 2013 Final Report Project SR12-002: Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary Gary L. Taghon, Rutgers University, Project Manager [email protected] Judith P. Grassle, Rutgers University, Co-Manager [email protected] Charlotte M. Fuller, Rutgers University, Co-Manager [email protected] Rosemarie F. Petrecca, Rutgers University, Co-Manager and Quality Assurance Officer [email protected] Patricia Ramey, Senckenberg Research Institute and Natural History Museum, Frankfurt Germany, Co-Manager [email protected] Thomas Belton, NJDEP Project Manager and NJDEP Research Coordinator [email protected] Marc Ferko, NJDEP Quality Assurance Officer [email protected] Bob Schuster, NJDEP Bureau of Marine Water Monitoring [email protected] Introduction The Barnegat Bay ecosystem is potentially under stress from human impacts, which have increased over the past several decades. Benthic macroinvertebrates are commonly included in studies to monitor the effects of human and natural stresses on marine and estuarine ecosystems. There are several reasons for this. Macroinvertebrates (here defined as animals retained on a 0.5-mm mesh sieve) are abundant in most coastal and estuarine sediments, typically on the order of 103 to 104 per meter squared. Benthic communities are typically composed of many taxa from different phyla, and quantitative measures of community diversity (e.g., Rosenberg et al. 2004) and the relative abundance of animals with different feeding behaviors (e.g., Weisberg et al. 1997, Pelletier et al. 2010), can be used to evaluate ecosystem health. Because most benthic invertebrates are sedentary as adults, they function as integrators, over periods of months to years, of the properties of their environment. -

Jacksonville, Florida 1998 Odmds Benthic Community Assessment
JACKSONVILLE, FLORIDA 1998 ODMDS BENTHIC COMMUNITY ASSESSMENT Submitted to U.S. Environmental Protection Agency, Region 4 61 Forsyth St. Atlanta, Georgia 30303 Prepared by Barry A. Vittor & Associates, Inc. 8060 Cottage Hill Rd. Mobile, Alabama 36695 (334) 633-6100 November 1999 TABLE OF CONTENTS LIST OF TABLES ………………………………………….……………………………3 LIST OF FIGURES ……………………..………………………………………………..4 1.0 INTRODUCTION ………..…………………………………………………………..5 2.0 METHODS ………..…………………………………………………………………..5 2.1 Sample Collection And Handling ………………………………………………5 2.2 Macroinfaunal Sample Analysis ……………………………………………….6 3.0 DATA ANALYSIS METHODS ……..………………………………………………6 3.1 Assemblage Analyses ..…………………………………………………………6 3.2 Faunal Similarities ……………………………………………………….…….8 4.0 HABITAT CHARACTERISTICS ……………………………………………….…8 5.0 BENTHIC COMMUNITY CHARACTERIZATION ……………………………..9 5.1 Faunal Composition, Abundance, And Community Structure …………………9 5.2 Numerical Classification Analysis …………………………………………….10 5.3 Taxa Assemblages …………………………………………………………….11 6.0 1995 vs 1998 COMPARISONS ……………………………………………………..11 7.0 SUMMARY ………………………………………………………………………….13 8.0 LITERATURE CITED ……………………………………………………………..16 2 LIST OF TABLES Table 1. Station locations for the Jacksonville, Florida ODMDS, June 1998. Table 2. Sediment data for the Jacksonville, Florida ODMDS, June 1998. Table 3. Summary of abundance of major taxonomic groups for the Jacksonville, Florida ODMDS, June 1998. Table 4. Abundance and distribution of major taxonomic groups at each station for the Jacksonville, Florida ODMDS, June 1998. Table 5. Abundance and distribution of taxa for the Jacksonville, Florida ODMDS, June 1998. Table 6. Percent abundance of dominant taxa (> 5% of the total assemblage) for the Jacksonville, Florida ODMDS, June 1998. Table 7. Summary of assemblage parameters for the Jacksonville, Florida ODMDS stations, June 1998. Table 8. Analysis of variance table for density differences between stations for the Jacksonville, Florida ODMDS stations, June 1998. -

Tube Epifaum of the Polychaete Phyllopchaetopterus Socialis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository Open Access to Scientific Information from Embrapa Estuarine, Coastal and Shelf Science (1995) 41, 91–100 Tube epifauna of the Polychaete Phyllochaetopterus socialis Claparède Rosebel Cunha Nalessoa, Luíz Francisco L. Duarteb, Ivo Pierozzi Jrc and Eloisa Fiorim Enumod aDepartamento de Zoologia, CCB, Universidade Federal de Pernambuco, 50670-901, Recife, PE, Brazil, bDepartamento de Zoologia, Instituto Biologia, C.P. 6109, Universidade Estadual de Campinas, 13.081-970, Campinas, SP, Brazil, cEmbrapa, NMA, Av. Dr. Julio Soares de Arruda, 803 CEP 13.085, Campinas, SP, Brazil and dProtebras, Rua Turmalina, 79 CEP 13.088, Campinas, SP, Brazil Received 8 October 1992 and in revised form 22 June 1994 Keywords: Polychaeta; tubes; faunal association; epifauna; São Sebastião Channel; Brazil Animals greater than 1 mm, found among tangled tubes of Phyllochaetopterus socialis (Chaetopteridae) from Araçá Beach, São Sebastião district, Brazil, were studied for 1 year, with four samples in each of four seasons. They comprised 10 338 individuals in 1722·7 g dry weight of polychaete tubes, with Echino- dermata, Polychaeta (not identified to species) and Crustacea as the dominant taxa. The Shannon–Wiener diversity index did not vary seasonally, only two species (a holothurian and a pycnogonid) showing seasonal variation. Ophiactis savignyi was the dominant species, providing 45·5% of individuals. Three other ophiuroids, the holothurian Synaptula hidriformis, the crustaceans Leptochelia savignyi, Megalobrachium soriatum and Synalpheus fritzmuelleri, the sipunculan Themiste alutacea and the bivalve Hiatella arctica were all abundant, but most of the 68 species recorded occurred sparsely. -

Foraging and Mobility in Three Species of Aciculata (Annelida: Polychaeta)
FORAGING AND MOBILITY IN THREE SPECIES OF ACICULATA (ANNELIDA: POLYCHAETA) PARDO, E. V. and AMARAL, A. C. Z. Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, CEP 13083-970, Campinas, São Paulo, Brazil Correspondence to: Erica Veronica Pardo, Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, CEP 13083-970, Campinas, São Paulo, Brazil, e-mail: [email protected] Received February 3, 2005 – Accepted August 22, 2005 – Distributed November 1, 2006 (With 1 figure) ABSTRACT Aspects of feeding, such as food capture and ingestion, as well as mobility of the polychaetes Eurythoe complanata, Marphysa formosa and Diopatra aciculata, from São Sebastião Channel (São Sebastião, state of São Paulo) were observed in laboratory conditions. Eurythoe complanata, a carnivorous species, fed exclusively on pieces of fish with the aid of strong muscular retractable lips, and detected the presence of food by chemical stimuli. Diopatra aciculata, an omnivorous species, captured and ingested different kinds of food with the aid of its jaws, generating a flow of water through its tube by which it detects the presence of food and oxygenates its gills. Marphysa formosa also used its jaws to bite and lacerate food. These species showed greater or lesser degrees of intolerance to light. Keywords: foraging, mobility, Aciculata, Polychaeta, Annelida. RESUMO Forrageamento e mobilidade em Polychaeta Alguns aspectos da atividade alimentar, tais como a captura e ingestão de alimento, bem como a mobilidade dos poliquetas Eurythoe complanata, Marphysa formosa e Diopatra aciculata, procedentes do Canal de São Sebastião (São Sebastião, SP), foram observados em laboratório. -

Benthic Habitats of the Delaware
BENTHICHABITATSOF THEDELAWAREBAY BenthicHabitatsoftheDelawareBay BenthicHabitatsofDelawareBay MarkG.Anderson,JosephA.M.Smith,andBartholomewD.Wilson INTRODUCTION ThissectiondescribesandmapsthemajorphysicalhabitatsoftheDelawareBayseafloor.Weused informationonbenthicorganisms,theirdistributionandtheirrelationshipstophysicalfeatures,to delimitadistinctsetofenvironmentsrepresentingthevarietyofbenthichabitatsintheBay.As individualspeciesareadaptedtovariationsindepth,sedimentsize,seabedtopographyandsalinity,we examinedthesefactorsinrelationshiptotheorganismcompositionandclassifiedthemintobasictypes toillustratethediversityofconditionsexistingontheseafloor.Wehopethatthisbenthichabitatmapof theDelawareBay,basedonpreviouslycollecteddata,willprovideabetterunderstandingofthe abundanceanddistributionofseafloorhabitattypes. Benthicorganismsarethosethatinhabittheseafloor;fromtheGreekwordbenthos,meaning“depths ofthesea.”Basedonajustasmallsample(246samples),theseafloorhabitatsoftheDelawareBay containover300speciesin8phylaincluding: 106speciesofarthropods(crabs,lobsters,shrimp,barnacles) 75speciesofmollusks(clams,scallops,squid,limpets,seaslugs,snails) 130speciesofannelids(seaworms) 8speciesofechinoderms(seastars,seaurchins,seacucumbers,sanddollars) 5speciesofcnidarians(corals,anemones,jellyfish) 4speciesofchordates(seasquirts) 1speciesofporiferans(sponges) 6speciesofnemerteans(ribbonworms) Thedistributionsandlifehistoriesofbenthicorganismsaretiedtotheirphysicalenvironment.Filter feederstendtodominateonshallowsandybottomswhiledepositfeeders,maydominateinfine -

Tube-Forming Polychaetes Enhance Invertebrate Diversity and Abundance in Sandy Sediments of Mozambique, Africa
African Journal of Marine Science 2011, 33(2): 327–332 Copyright © NISC (Pty) Ltd Printed in South Africa — All rights reserved AFRICAN JOURNAL OF MARINE SCIENCE ISSN 1814–232X EISSN 1814–2338 doi: 10.2989/1814232X.2011.600433 Short Communication Tube-forming polychaetes enhance invertebrate diversity and abundance in sandy sediments of Mozambique, Africa MS Thomsen1,2*, MF Muth3 and KJ McGlathery3 1 Marine Department, National Environmental Research Institute, University of Aarhus, PO Box 4000, Roskilde, Denmark 2 School of Plant Biology, University of Western Australia, Crawley 6009 WA, Australia 3 Department of Environmental Sciences, University of Virginia, 291 McCormick Rd, Clark Hall, Charlottesville, VA 22904, USA * Corresponding author, e-mail: [email protected] Manuscript received March 2011; accepted May 2011 In marine soft-bottom systems, polychaetes can increase habitat complexity by constructing rigid tubes (e.g. several onuphid species) that contrast with surrounding topographically flat sediments. These structures can provide predation refuges and increase larval settlement and thereby increase the richness and abundance of fauna. We collected invertebrate samples from an intertidal flat with low onuphid tube density (2.7 m–2) in Mozambique and document that more organisms (70 times higher mollusc abundances) and more species (15 times more mollusc species) were found associated with solitary tubes of an onuphid polychaete compared with surrounding sand habitats. These results are in agreement with tube versus sand comparisons from soft-bottom systems in the North Atlantic where polychaete tube densities are often much higher. Keywords: habitat formation, onuphid polychaete, species richness, western Indian Ocean Introduction Species that form or modify habitat, often referred to as (Thomsen et al. -

Initial Survey of Plum Island's Marine Habitats
Initial Survey of Plum Island’s Marine Habitats New York Natural Heritage Program Initial Survey of Plum Island’s Marine Habitats Emily S. Runnells Matthew D. Schlesinger Gregory J. Edinger New York Natural Heritage Program and Steven C. Resler Dan Marelli InnerSpace Scientific Diving A report to Save the Sound April 2020 Please cite this report as follows: New York Natural Heritage Program and InnerSpace Scientific Diving. 2020. Initial survey of Plum Island’s marine habitats. Report to Save the Sound. Available from New York Natural Heritage Program, Albany, NY. Available at www.nynhp.org/plumisland. Cover photos (left to right, top to bottom): Bryozoans and sponges; lion’s mane jellyfish; flat-clawed hermit crab; diver recording information from inside quadrat; bryozoans, sponges and northern star corals. All photos herein by the authors. Contents Introduction ........................................................................................................................................................ 1 Methods ............................................................................................................................................................... 1 Results .................................................................................................................................................................. 6 Discussion and Next Steps ............................................................................................................................... 9 Acknowledgments........................................................................................................................................... -

Some Intertidal and Shallow Water Polychaetes of the Caribbean Coast of Costa Rica
Some intertidal and shallow water polychaetes of the Caribbean coast of Costa Rica Harlan K. Dean1, 2 1. Department of Biology, University of Massachusetts- Boston, Boston, Massachusetts 02125-3393, USA; [email protected] 2. Department of Invertebrates, Museum of Comparative Zoology, Harvard University, 26 Oxford Street, Cambridge, Massachusetts 02138, USA Received 09-VII-2016. Corrected 06-IX-2016. Accepted 07-X-2016. Abstract: The polychaete fauna of the Caribbean coast of Costa Rica has been inadequately characterized with only nine species previously reported. Collections of polychaetes from intertidal coralline rocks and several shal- low sub-tidal sites on the Caribbean coast of Costa Rica have been examined and 68 species were identified. Of these, 66 are new records for Costa Rica. Rev. Biol. Trop. 65 (1): 127-152. Epub 2017 March 01. Key words: Annelida, Polychaeta, Caribbean Sea, Costa Rica, marine biodiversity, intertidal. The Caribbean coast of Costa Rica is species from the rocky intertidal of Cahuita. shorter (212 km) when compared with its Nonetheless, the occurrence of only nine known Pacific Coast (1 254 km) (Cortés & Wehrt- species of polychaetes indicates that this taxon mann, 2009). Much of the Caribbean coast is has been largely neglected in this region. sandy beaches with some rocky areas and coral In this paper materials collected during reefs in the Southern portion. Sea-grass beds several trips to the Caribbean coast of Costa also occur in the lagoons of the coral reefs and Rica funded by Centro de Investigación en consist mainly of Thalassia testidunum with Ciencias del Mar y Limnología (CIMAR), some Syringodium filiformis interspersed (Cor- University of Costa Rica (UCR), were ana- tés & Wehrtmann, 2009). -
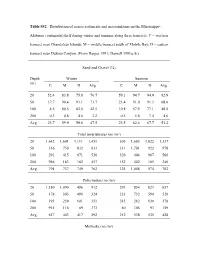
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg. -

Polychaete Diversity in the Estuarine Habitats of Términos Lagoon, Southern Gulf of Mexico
Memoirs of Museum Victoria 71: 97–107 (2014) Published December 2014 ISSN 1447-2546 (Print) 1447-2554 (On-line) http://museumvictoria.com.au/about/books-and-journals/journals/memoirs-of-museum-victoria/ Polychaete diversity in the estuarine habitats of Términos Lagoon, southern Gulf of Mexico PABLO HERNÁNDEZ-ALCÁNTARA1 (http://zoobank.org/urn:lsid:zoobank.org:author:6DA0DE56-E980-4B31-9B16-BEBAA35DE639), J. DANIEL CORTÉS-SOLANO2 (http://zoobank.org/urn:lsid:zoobank.org:author:C32969C6-5213-4903-9F96-27A37ED24286), NADIEZHDA M. MEDINA-CANTÚ3 (http://zoobank.org/urn:lsid:zoobank.org:author:1082E3F0-8AC4-4091-8948-45C1685C50E8), A. LAURA AVILÉS-DÍAZ4 (http://zoobank.org/urn:lsid:zoobank.org:author:E18A5F35-9826-4E83-876D-3258D76FEFDF) AND VIVIANNE SOLÍS-WEISS*,5 (http://zoobank.org/urn:lsid:zoobank.org:author:9D486357-8D55-4B9B-8A04-CE3912359848) 1, 2, 3, 4 Unidad Académica de Ecología y Biodiversidad Acuática, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Circuito Exterior S/N. Cd. Universitaria, D. F. 04510, México (1 [email protected]; 2 [email protected]; 3 [email protected]; 4 [email protected]) 5 Unidad Académica Sistemas Arrecifales Puerto Morelos, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México. Puerto Morelos, Q. Roo, 77580, México ([email protected]) * To whom correspondence and reprint requests should be addressed. Email: [email protected] http://zoobank.org/urn:lsid:zoobank.org:pub:599BB612-1816-4584-90EA-37104C4501C9 Abstract Hernández-Alcántara, P., Cortés-Solano, J.D., Medina-Cantú, N.M., Avilés-Díaz, A.L. and Solís-Weiss, V. 2014. -

Diopatra Cuprea
MARINE ECOLOGY PROGRESS SERIES Vol. 183: 169-178.1999 Published July 6 1 Mar Ecol Prog Ser l Distributions of total and active bacteria in biofilms lining tubes of the onuphid polychaete Diopatra cuprea Tina M. Phillips, Charles R. Lovell* Department of Biological Sciences, University of South Carolina, Columbia, South Carolina 29208, USA ABSTRACT: Infaunal burrows and tubes support elevated levels of microbial biomass and activities relative to the surrounding bulk sediments. The impacts of these structures on sediment biogeochem- istry have been intensively studled, but very little is known about the microbiota or their spatial orga- nization within these microenvironments. Distributions of bacterial cells and microcolonies and of potentially active bacterial cells in tubes of the onuphid polychaete Diopatra cuprea were determined using confocal scanning laser microscopy. Distributions were examined among different tubes, at dif- ferent locations within a tube, and at different depths in the biofilms lining these tubes. The average quantity of cells across all tubes examined was 5.61 X 10' cm-3 and there was no trend in the distribu- tion of cells along the length of the tube or with increasing depth in the biofilm. Cell size across all tubes collected in February 1997 averaged 0.085 pm3 but varied seasonally. Mean cell size increased with increasing depth below the sediment surface of the tube site sampled, and decreased with increasing depth in the biofilm. Microcolonies were abundant and not confined to specific depths within the biofh or locations within the tube. Potentially active cells were observed at all depths within the biofilm and at least 46% (mean = 68%) of cells at any given site were potentially active. -

GC .S6 N~G ·E;~~ :.~"
GC 1000 .S6 n~g ·e;~~ :.~" GC 1000 .D65 E34 1979 EFFECTS OF DREDGING AND UNCONFINED DISPOSAL OF DREDGED MATERIAL ON MACROBENTHIC COMMUNITIES IN SEWEE BAY, SOUTH CAROLINAl by Robert F. Van Dolah Dale R. Calder David M. Knott Magdalene S. Maclin Marine Resources Center South Carolina Wildlife and Marine Resources Department Charleston, South Carolina 29412 Technical Report No. 39 South Carolina Marine Resources Center April 1979 Proper~y of csc Library 1 Study funded under contract (#DACW-60-77-C-0013) for the U. S. Army Corps of Engineers, Charleston District. US Department of Commerce NOP.A co~1.::.; tal Services Center Library 2234 South Hobson Avenue Charleston, SC 29405-2413 TABLE OF CONTENTS Page LIST OF FIGURES •••••••..••••......•.••••••••.••.•.••..•.••••..•.••.••••..•••••.•....•••••....•••••••..... iii LIST OF TABLES........................................................ iii INTRODUCTION. • • • • • . • . • • • • • . • • • . • . • . • . • . • • . • . • . 1 DESCRIPTION OF STUDY AREA. 1 METHODS.................................................................................................. 1 Benthic Sampling.................................................................................... 1 Benthic Analytical Techniques....................................................................... 1 Water Chemistry................................................. • • • . • • . • . • 3 RESULTS AND DISCUSSION................................................................................... 3 Water Chemistry, ••••••••• ,, •••••• ,,,,,,,