Some Intertidal and Shallow Water Polychaetes of the Caribbean Coast of Costa Rica
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Full Curriculum Vitae
C. R. Smith July 2017 Curriculum Vitae CRAIG RANDALL SMITH Address: Department of Oceanography University of Hawaii at Manoa 1000 Pope Road Honolulu, HI 96822 Telephone: 808-956-7776 email: [email protected] Education: B.S., 1977, with high honors, Biological Science, Michigan State University Ph.D., Dec 1983, Biological Oceanography, University of California at San Diego, Scripps Institution of Oceanography Professional Experience: 1975-1976: Teaching Assistant, Biological Science Program, Michigan State University 1976: Summer Student Fellow, Woods Hole Oceanographic Institution 1976-1977: Research Assistant, Microbiology Department, Michigan State University 1977-1981: Research Assistant, Program for the Study of Sub- Seabed Disposal of Radioactive Waste, Scripps Institution of Oceanography 1981-1983: Associate Investigator, O.N.R. grant entitled, "The Impact of Large Organic Falls on a Bathyal Benthic Community," Scripps Institution of Oceanography 1983-1984: Postdoctoral Scholar, Woods Hole Oceanographic Institution 1985-1986: Postdoctoral Research Associate, School of Oceanography, University of Washington 1986-1988: Research Assistant Professor, School of Oceanography, University of Washington 1988-1995: Associate Professor, Department of Oceanography, University of Hawaii at Manoa 1995-1998, 2004-2007: Chair, Biological Oceanography Division, University of Hawaii at Manoa 1997-1998, 2006-2007: Associate Chair, Department of Oceanography 1995-present: Professor, Department of Oceanography, University of Hawaii at Manoa Major Research -

Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary
July 15, 2013 Final Report Project SR12-002: Benthic Invertebrate Community Monitoring and Indicator Development for Barnegat Bay-Little Egg Harbor Estuary Gary L. Taghon, Rutgers University, Project Manager [email protected] Judith P. Grassle, Rutgers University, Co-Manager [email protected] Charlotte M. Fuller, Rutgers University, Co-Manager [email protected] Rosemarie F. Petrecca, Rutgers University, Co-Manager and Quality Assurance Officer [email protected] Patricia Ramey, Senckenberg Research Institute and Natural History Museum, Frankfurt Germany, Co-Manager [email protected] Thomas Belton, NJDEP Project Manager and NJDEP Research Coordinator [email protected] Marc Ferko, NJDEP Quality Assurance Officer [email protected] Bob Schuster, NJDEP Bureau of Marine Water Monitoring [email protected] Introduction The Barnegat Bay ecosystem is potentially under stress from human impacts, which have increased over the past several decades. Benthic macroinvertebrates are commonly included in studies to monitor the effects of human and natural stresses on marine and estuarine ecosystems. There are several reasons for this. Macroinvertebrates (here defined as animals retained on a 0.5-mm mesh sieve) are abundant in most coastal and estuarine sediments, typically on the order of 103 to 104 per meter squared. Benthic communities are typically composed of many taxa from different phyla, and quantitative measures of community diversity (e.g., Rosenberg et al. 2004) and the relative abundance of animals with different feeding behaviors (e.g., Weisberg et al. 1997, Pelletier et al. 2010), can be used to evaluate ecosystem health. Because most benthic invertebrates are sedentary as adults, they function as integrators, over periods of months to years, of the properties of their environment. -

Jacksonville, Florida 1998 Odmds Benthic Community Assessment
JACKSONVILLE, FLORIDA 1998 ODMDS BENTHIC COMMUNITY ASSESSMENT Submitted to U.S. Environmental Protection Agency, Region 4 61 Forsyth St. Atlanta, Georgia 30303 Prepared by Barry A. Vittor & Associates, Inc. 8060 Cottage Hill Rd. Mobile, Alabama 36695 (334) 633-6100 November 1999 TABLE OF CONTENTS LIST OF TABLES ………………………………………….……………………………3 LIST OF FIGURES ……………………..………………………………………………..4 1.0 INTRODUCTION ………..…………………………………………………………..5 2.0 METHODS ………..…………………………………………………………………..5 2.1 Sample Collection And Handling ………………………………………………5 2.2 Macroinfaunal Sample Analysis ……………………………………………….6 3.0 DATA ANALYSIS METHODS ……..………………………………………………6 3.1 Assemblage Analyses ..…………………………………………………………6 3.2 Faunal Similarities ……………………………………………………….…….8 4.0 HABITAT CHARACTERISTICS ……………………………………………….…8 5.0 BENTHIC COMMUNITY CHARACTERIZATION ……………………………..9 5.1 Faunal Composition, Abundance, And Community Structure …………………9 5.2 Numerical Classification Analysis …………………………………………….10 5.3 Taxa Assemblages …………………………………………………………….11 6.0 1995 vs 1998 COMPARISONS ……………………………………………………..11 7.0 SUMMARY ………………………………………………………………………….13 8.0 LITERATURE CITED ……………………………………………………………..16 2 LIST OF TABLES Table 1. Station locations for the Jacksonville, Florida ODMDS, June 1998. Table 2. Sediment data for the Jacksonville, Florida ODMDS, June 1998. Table 3. Summary of abundance of major taxonomic groups for the Jacksonville, Florida ODMDS, June 1998. Table 4. Abundance and distribution of major taxonomic groups at each station for the Jacksonville, Florida ODMDS, June 1998. Table 5. Abundance and distribution of taxa for the Jacksonville, Florida ODMDS, June 1998. Table 6. Percent abundance of dominant taxa (> 5% of the total assemblage) for the Jacksonville, Florida ODMDS, June 1998. Table 7. Summary of assemblage parameters for the Jacksonville, Florida ODMDS stations, June 1998. Table 8. Analysis of variance table for density differences between stations for the Jacksonville, Florida ODMDS stations, June 1998. -

Revision of Hyalopale (Chrysopetalidae; Phyllodocida; Annelida
Revision of Hyalopale (Chrysopetalidae; Phyllodocida; Annelida) an amphi-Atlantic Hyalopale bispinosa species complex and five new species from reefs of the Caribbean Sea and Indo-Pacific Oceans Watson, Charlotte; Tilic, Ekin; Rouse, Greg W. Published in: Zootaxa DOI: 10.11646/zootaxa.4671.3.2 Publication date: 2019 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Watson, C., Tilic, E., & Rouse, G. W. (2019). Revision of Hyalopale (Chrysopetalidae; Phyllodocida; Annelida): an amphi-Atlantic Hyalopale bispinosa species complex and five new species from reefs of the Caribbean Sea and Indo-Pacific Oceans. Zootaxa, 4671(3), 339-368. https://doi.org/10.11646/zootaxa.4671.3.2 Download date: 27. sep.. 2021 Zootaxa 4671 (3): 339–368 ISSN 1175-5326 (print edition) https://www.mapress.com/j/zt/ Article ZOOTAXA Copyright © 2019 Magnolia Press ISSN 1175-5334 (online edition) https://doi.org/10.11646/zootaxa.4671.3.2 http://zoobank.org/urn:lsid:zoobank.org:pub:99459D5F-3C35-4F7D-9768-D70616676851 Revision of Hyalopale (Chrysopetalidae; Phyllodocida; Annelida): an amphi-Atlantic Hyalopale bispinosa species complex and five new species from reefs of the Caribbean Sea and Indo-Pacific Oceans CHARLOTTE WATSON1, EKIN TILIC2 & GREG W. ROUSE2 1Museum & Art Gallery of the Northern Territory, Box 4646, Darwin, 0801 NT, Australia. E-mail: [email protected] 2Scripps Institution of Oceanography, UC San Diego, La Jolla, CA 92093-0202, USA. E-mail: [email protected] Abstract The formerly monotypic taxon, Hyalopale bispinosa Perkins 1985 (Chrysopetalinae), is comprised of a cryptic species complex from predominantly tropical embayments and island reefs of the Western Atlantic and Indo-Pacific Oceans. -

Tube Epifaum of the Polychaete Phyllopchaetopterus Socialis
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository Open Access to Scientific Information from Embrapa Estuarine, Coastal and Shelf Science (1995) 41, 91–100 Tube epifauna of the Polychaete Phyllochaetopterus socialis Claparède Rosebel Cunha Nalessoa, Luíz Francisco L. Duarteb, Ivo Pierozzi Jrc and Eloisa Fiorim Enumod aDepartamento de Zoologia, CCB, Universidade Federal de Pernambuco, 50670-901, Recife, PE, Brazil, bDepartamento de Zoologia, Instituto Biologia, C.P. 6109, Universidade Estadual de Campinas, 13.081-970, Campinas, SP, Brazil, cEmbrapa, NMA, Av. Dr. Julio Soares de Arruda, 803 CEP 13.085, Campinas, SP, Brazil and dProtebras, Rua Turmalina, 79 CEP 13.088, Campinas, SP, Brazil Received 8 October 1992 and in revised form 22 June 1994 Keywords: Polychaeta; tubes; faunal association; epifauna; São Sebastião Channel; Brazil Animals greater than 1 mm, found among tangled tubes of Phyllochaetopterus socialis (Chaetopteridae) from Araçá Beach, São Sebastião district, Brazil, were studied for 1 year, with four samples in each of four seasons. They comprised 10 338 individuals in 1722·7 g dry weight of polychaete tubes, with Echino- dermata, Polychaeta (not identified to species) and Crustacea as the dominant taxa. The Shannon–Wiener diversity index did not vary seasonally, only two species (a holothurian and a pycnogonid) showing seasonal variation. Ophiactis savignyi was the dominant species, providing 45·5% of individuals. Three other ophiuroids, the holothurian Synaptula hidriformis, the crustaceans Leptochelia savignyi, Megalobrachium soriatum and Synalpheus fritzmuelleri, the sipunculan Themiste alutacea and the bivalve Hiatella arctica were all abundant, but most of the 68 species recorded occurred sparsely. -

<I>Diopatra Cuprea</I>
BULLETIN OF MARINE SCIENCE, 40(1): 11-21, 1987 ROLE OF DIOPATRA CUPREA BOSC (POLYCHAETA: ONUPHIDAE) TUBES IN STRUCTURING A SUBTROPICAL INFAUNAL COMMUNITY Suzanne M. Ban and Walter G. Nelson ABSTRACT An a priori hypothesis predicted that in the vicinity of aggregated Diopatra cuprea tubes an enhanced infaunal density and species richness would be found, resulting from a biological refuge effect of the tubes. To test this hypothesis, cores were taken over a 5-month period in both vegetated, Halodule wrighti! Aschers. beds, and unvegetated areas of a site in the Indian River lagoon, Florida. An inner, 0.01 m2, frame was placed to enclose densities of 0, I, or 4 D. cuprea tubes, while an outer concentric, 0.02 m2, frame was placed so that it enclosed the smaller frame, plus a surrounding area lacking in D. cuprea tubes. The presence of D. cuprea tubes was found to have no consistent significant effect on the abundance and number of infaunal species found in either the vegetated or unvegetated areas. Laboratory experiments employing a benthic predator, Callinectes, were carried out in order to determine whether D. cuprea tubes andlor H. wrightii rhizome mats actually constitute a barrier to predation. Significantly higher survivorship of the bivalve Mulinia lateralis Say, used as prey, was found in laboratory treatments containing 10 tubes per 0.01 m2 versus treatments containing 4 or a tubes per 0.01 m2. Highest survivorship of bivalves was found in treatments containing a H. wrightii rhizome mat; tubes placed within the mat did not enhance clam survivorship. The discrepancy between the findings of this study, and previous studies on the refuge effect of D. -

Foraging and Mobility in Three Species of Aciculata (Annelida: Polychaeta)
FORAGING AND MOBILITY IN THREE SPECIES OF ACICULATA (ANNELIDA: POLYCHAETA) PARDO, E. V. and AMARAL, A. C. Z. Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, CEP 13083-970, Campinas, São Paulo, Brazil Correspondence to: Erica Veronica Pardo, Departamento de Zoologia, Instituto de Biologia, Universidade Estadual de Campinas, C. P. 6109, CEP 13083-970, Campinas, São Paulo, Brazil, e-mail: [email protected] Received February 3, 2005 – Accepted August 22, 2005 – Distributed November 1, 2006 (With 1 figure) ABSTRACT Aspects of feeding, such as food capture and ingestion, as well as mobility of the polychaetes Eurythoe complanata, Marphysa formosa and Diopatra aciculata, from São Sebastião Channel (São Sebastião, state of São Paulo) were observed in laboratory conditions. Eurythoe complanata, a carnivorous species, fed exclusively on pieces of fish with the aid of strong muscular retractable lips, and detected the presence of food by chemical stimuli. Diopatra aciculata, an omnivorous species, captured and ingested different kinds of food with the aid of its jaws, generating a flow of water through its tube by which it detects the presence of food and oxygenates its gills. Marphysa formosa also used its jaws to bite and lacerate food. These species showed greater or lesser degrees of intolerance to light. Keywords: foraging, mobility, Aciculata, Polychaeta, Annelida. RESUMO Forrageamento e mobilidade em Polychaeta Alguns aspectos da atividade alimentar, tais como a captura e ingestão de alimento, bem como a mobilidade dos poliquetas Eurythoe complanata, Marphysa formosa e Diopatra aciculata, procedentes do Canal de São Sebastião (São Sebastião, SP), foram observados em laboratório. -

Benthic Habitats of the Delaware
BENTHICHABITATSOF THEDELAWAREBAY BenthicHabitatsoftheDelawareBay BenthicHabitatsofDelawareBay MarkG.Anderson,JosephA.M.Smith,andBartholomewD.Wilson INTRODUCTION ThissectiondescribesandmapsthemajorphysicalhabitatsoftheDelawareBayseafloor.Weused informationonbenthicorganisms,theirdistributionandtheirrelationshipstophysicalfeatures,to delimitadistinctsetofenvironmentsrepresentingthevarietyofbenthichabitatsintheBay.As individualspeciesareadaptedtovariationsindepth,sedimentsize,seabedtopographyandsalinity,we examinedthesefactorsinrelationshiptotheorganismcompositionandclassifiedthemintobasictypes toillustratethediversityofconditionsexistingontheseafloor.Wehopethatthisbenthichabitatmapof theDelawareBay,basedonpreviouslycollecteddata,willprovideabetterunderstandingofthe abundanceanddistributionofseafloorhabitattypes. Benthicorganismsarethosethatinhabittheseafloor;fromtheGreekwordbenthos,meaning“depths ofthesea.”Basedonajustasmallsample(246samples),theseafloorhabitatsoftheDelawareBay containover300speciesin8phylaincluding: 106speciesofarthropods(crabs,lobsters,shrimp,barnacles) 75speciesofmollusks(clams,scallops,squid,limpets,seaslugs,snails) 130speciesofannelids(seaworms) 8speciesofechinoderms(seastars,seaurchins,seacucumbers,sanddollars) 5speciesofcnidarians(corals,anemones,jellyfish) 4speciesofchordates(seasquirts) 1speciesofporiferans(sponges) 6speciesofnemerteans(ribbonworms) Thedistributionsandlifehistoriesofbenthicorganismsaretiedtotheirphysicalenvironment.Filter feederstendtodominateonshallowsandybottomswhiledepositfeeders,maydominateinfine -

Tube-Forming Polychaetes Enhance Invertebrate Diversity and Abundance in Sandy Sediments of Mozambique, Africa
African Journal of Marine Science 2011, 33(2): 327–332 Copyright © NISC (Pty) Ltd Printed in South Africa — All rights reserved AFRICAN JOURNAL OF MARINE SCIENCE ISSN 1814–232X EISSN 1814–2338 doi: 10.2989/1814232X.2011.600433 Short Communication Tube-forming polychaetes enhance invertebrate diversity and abundance in sandy sediments of Mozambique, Africa MS Thomsen1,2*, MF Muth3 and KJ McGlathery3 1 Marine Department, National Environmental Research Institute, University of Aarhus, PO Box 4000, Roskilde, Denmark 2 School of Plant Biology, University of Western Australia, Crawley 6009 WA, Australia 3 Department of Environmental Sciences, University of Virginia, 291 McCormick Rd, Clark Hall, Charlottesville, VA 22904, USA * Corresponding author, e-mail: [email protected] Manuscript received March 2011; accepted May 2011 In marine soft-bottom systems, polychaetes can increase habitat complexity by constructing rigid tubes (e.g. several onuphid species) that contrast with surrounding topographically flat sediments. These structures can provide predation refuges and increase larval settlement and thereby increase the richness and abundance of fauna. We collected invertebrate samples from an intertidal flat with low onuphid tube density (2.7 m–2) in Mozambique and document that more organisms (70 times higher mollusc abundances) and more species (15 times more mollusc species) were found associated with solitary tubes of an onuphid polychaete compared with surrounding sand habitats. These results are in agreement with tube versus sand comparisons from soft-bottom systems in the North Atlantic where polychaete tube densities are often much higher. Keywords: habitat formation, onuphid polychaete, species richness, western Indian Ocean Introduction Species that form or modify habitat, often referred to as (Thomsen et al. -

OREGON ESTUARINE INVERTEBRATES an Illustrated Guide to the Common and Important Invertebrate Animals
OREGON ESTUARINE INVERTEBRATES An Illustrated Guide to the Common and Important Invertebrate Animals By Paul Rudy, Jr. Lynn Hay Rudy Oregon Institute of Marine Biology University of Oregon Charleston, Oregon 97420 Contract No. 79-111 Project Officer Jay F. Watson U.S. Fish and Wildlife Service 500 N.E. Multnomah Street Portland, Oregon 97232 Performed for National Coastal Ecosystems Team Office of Biological Services Fish and Wildlife Service U.S. Department of Interior Washington, D.C. 20240 Table of Contents Introduction CNIDARIA Hydrozoa Aequorea aequorea ................................................................ 6 Obelia longissima .................................................................. 8 Polyorchis penicillatus 10 Tubularia crocea ................................................................. 12 Anthozoa Anthopleura artemisia ................................. 14 Anthopleura elegantissima .................................................. 16 Haliplanella luciae .................................................................. 18 Nematostella vectensis ......................................................... 20 Metridium senile .................................................................... 22 NEMERTEA Amphiporus imparispinosus ................................................ 24 Carinoma mutabilis ................................................................ 26 Cerebratulus californiensis .................................................. 28 Lineus ruber ......................................................................... -

Initial Survey of Plum Island's Marine Habitats
Initial Survey of Plum Island’s Marine Habitats New York Natural Heritage Program Initial Survey of Plum Island’s Marine Habitats Emily S. Runnells Matthew D. Schlesinger Gregory J. Edinger New York Natural Heritage Program and Steven C. Resler Dan Marelli InnerSpace Scientific Diving A report to Save the Sound April 2020 Please cite this report as follows: New York Natural Heritage Program and InnerSpace Scientific Diving. 2020. Initial survey of Plum Island’s marine habitats. Report to Save the Sound. Available from New York Natural Heritage Program, Albany, NY. Available at www.nynhp.org/plumisland. Cover photos (left to right, top to bottom): Bryozoans and sponges; lion’s mane jellyfish; flat-clawed hermit crab; diver recording information from inside quadrat; bryozoans, sponges and northern star corals. All photos herein by the authors. Contents Introduction ........................................................................................................................................................ 1 Methods ............................................................................................................................................................... 1 Results .................................................................................................................................................................. 6 Discussion and Next Steps ............................................................................................................................... 9 Acknowledgments........................................................................................................................................... -
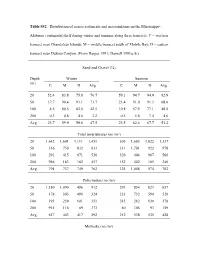
Table S32. Distribution of Coarse Sediments and Macroinfauna on the Mississippi
Table S32. Distribution of coarse sediments and macroinfauna on the Mississippi- Alabama continental shelf during winter and summer along three transects. C = western transect near Chandeleur Islands; M = middle transect south of Mobile Bay; D = eastern transect near DeSoto Canyon. (From Harper 1991; Darnell 1991a, b.) Sand and Gravel (%) Depth Winter Summer (m) C M D Avg. C M D Avg. 20 52.4 83.8 75.8 70.7 59.1 94.7 94.9 82.9 50 37.7 90.4 93.1 73.7 23.4 91.0 91.3 68.6 100 4.5 60.5 62.6 42.5 10.8 57.9 77.1 48.6 200 0.3 4.8 4.6 3.2 0.5 5.8 7.4 4.6 Avg. 23.7 59.9 59.0 47.5 23.5 62.4 67.7 51.2 Total invertebrates (no./m²) 20 1,642 1,601 1,111 1,451 505 1,683 1,822 1,337 50 316 750 832 633 311 1,701 922 978 100 291 415 871 526 326 404 967 566 200 946 183 182 457 152 402 185 246 Avg. 794 737 749 762 324 1,048 974 782 Polychaetes (no./m²) 20 1,180 1,090 486 912 293 854 823 657 50 178 305 490 324 233 732 594 520 100 193 259 601 351 243 262 630 378 200 915 116 89 373 80 305 93 159 Avg. 617 443 417 492 212 538 535 428 Mollusks (no./m²) 20 123 127 174 141 89 527 407 341 50 46 214 115 125 11 653 73 246 100 32 80 23 45 20 63 38 40 200 14 7 10 10 26 27 9 21 Avg.