The Ecological Factors Governing the Persistence of Butterflies in Urban Areas
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

General Methods Will Be Outlined in Chapter 2
The Biodiversity of Abandoned Farmland Tom Fayle Gonville and Caius College April 2005 This dissertation is submitted for the degree of Master of Philosophy Mating Six-spot Burnet moths (Zygaena filipendulae) on the Roughs Declaration This dissertation is the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. This dissertation does not exceed the limit of 15000 words in the main text, excluding figures, tables, legends and appendices. i Acknowledgements This work was carried out on the land of Miriam Rothschild, who sadly passed away before its completion. I would like to thank her for allowing me to stay at Ashton Wold during my fieldwork and making me feel welcome there. I would also like to thank the Eranda Foundation for funding this work. Various people have helped with the identification of my material and I am very grateful to them for their time. Brian Eversham was of great help in identifying my carabids and also took time out from his busy schedule to assist me for a day during my time in the field. Ray Symonds dedicated a great deal of time to identifying all the spiders I caught, a feat which would have undoubtedly taken me many weeks! Richard Preece identified all my gastropods, and I am grateful both to him and his student George Speller for passing on the material to him. Roger Morris verified the identification of voucher specimens of all the syrphids I caught, and Oliver Prŷs-Jones did the same for my bumblebees. -

Additions, Deletions and Corrections to An
Bulletin of the Irish Biogeographical Society No. 36 (2012) ADDITIONS, DELETIONS AND CORRECTIONS TO AN ANNOTATED CHECKLIST OF THE IRISH BUTTERFLIES AND MOTHS (LEPIDOPTERA) WITH A CONCISE CHECKLIST OF IRISH SPECIES AND ELACHISTA BIATOMELLA (STAINTON, 1848) NEW TO IRELAND K. G. M. Bond1 and J. P. O’Connor2 1Department of Zoology and Animal Ecology, School of BEES, University College Cork, Distillery Fields, North Mall, Cork, Ireland. e-mail: <[email protected]> 2Emeritus Entomologist, National Museum of Ireland, Kildare Street, Dublin 2, Ireland. Abstract Additions, deletions and corrections are made to the Irish checklist of butterflies and moths (Lepidoptera). Elachista biatomella (Stainton, 1848) is added to the Irish list. The total number of confirmed Irish species of Lepidoptera now stands at 1480. Key words: Lepidoptera, additions, deletions, corrections, Irish list, Elachista biatomella Introduction Bond, Nash and O’Connor (2006) provided a checklist of the Irish Lepidoptera. Since its publication, many new discoveries have been made and are reported here. In addition, several deletions have been made. A concise and updated checklist is provided. The following abbreviations are used in the text: BM(NH) – The Natural History Museum, London; NMINH – National Museum of Ireland, Natural History, Dublin. The total number of confirmed Irish species now stands at 1480, an addition of 68 since Bond et al. (2006). Taxonomic arrangement As a result of recent systematic research, it has been necessary to replace the arrangement familiar to British and Irish Lepidopterists by the Fauna Europaea [FE] system used by Karsholt 60 Bulletin of the Irish Biogeographical Society No. 36 (2012) and Razowski, which is widely used in continental Europe. -

Jan to Jun 2011
Butterfly Conservation Hampshire and Isle of Wight Branch Page 1 of 18 Butterfly Conservation Hampshire and Saving butterflies, moths and our environment Isle of Wight Branch HOME ABOUT US EVENTS CONSERVATION HANTS & IOW SPECIES SIGHTINGS PUBLICATIONS LINKS MEMBER'S AREA Thursday 30th June Christine Reeves reports from Ash Lock Cottage (SU880517) where the following observations were made: Purple Emperor (1 "Rather battered specimen"). "Following the excitement of seeing our first Purple Emperor inside our office yesterday, exactly the same thing happened again today at around 9.45am. The office door was open and we spotted a butterfly on the inside of the window, on closer inspection we realised it was a Purple Emperor. It was much smaller than the one we had seen the day before and more battered. However we were able to take pictures of it, in fact the butterfly actually climbed onto one of the cameras and remained there for a while. It then climbed from camera to hand, and we took it outside for more pictures before it eventually flew off. It seemed to be feeding off the hand.". Purple Empeor Purple Empeor Terry Hotten writes: "A brief walk around Hazeley Heath this morning produced a fresh Small Tortoiseshell along with Marbled Whites, Silver- studded Blues in reasonable numbers along with Meadow Browns, Ringlets and Large and Small Skippers." peter gardner reports from highcross froxfield (SU712266) where the following observations were made: Red Admiral (1 "purched on an hot window "). Red Admiral (RWh) Bob Whitmarsh reports from Plague Pits Valley, St Catherine's Hill (SU485273) where the following observations were made: Marbled White (23), Meadow Brown (41), Small Heath (7), Small Skipper (2), Ringlet (2), Red Admiral (3), Small Tortoiseshell (4), Small White (2), Comma (1). -

MMBG Newsletter No.84
MONMOUTHSHIRE MOTH & BUTTERFLY GROUP NEWSLETTER No 84 June 2012. A monthly newsletter covering Gwent and Monmouthshire Vice County 35 Editor: Martin Anthoney Monmouthshire Microlepidoptera - Changes to the county list since 1994 Sam Bosanquet Following Sam’s article in last month’s issue (No 83) on changes to the VC35 county micromoth list since the publication of Neil Horton’s book (Monmouthshire Lepidoptera, the Butterflies and Moths of Gwent) in 1994, attached to this newsletter as a separate file are details of the 181 micro species added to the list in the 18 years since the book’s publication. Scarce Bordered Straw (Helicoverpa armigera) This immigrant moth occurs in Britain most years but is usually scarce, though 300 were recorded in 1996. One of our members, Bob Roome, recalls his encounters with Scarce Bordered Straw in Africa:- I was introduced to the species in Botswana in 1968, as part of an Overseas Development Administration entomologist team from the Centre for Overseas Pest Research (London). As my first job, I accompanied two experienced entomologists charged with surveying the insect pests of crops in Botswana, carrying out research into their biology and control and advising farmers. We were effectively the first entomologists in the country and quickly set up the beginnings of a national collection in our new research station. Despite being a semi-arid country, merging into the Kalahari Desert, Botswana had a rich insect fauna. “Scarce Bordered Straw” was certainly not scarce where we worked. From the survey, it was clear that the “American Bollworm”, as H. armigera was known, was a major pest. -

Butterflies and Day Flying Moths of the Malvern Hills
Butterflies and Day andDay Butterflies the Malvern Hills the Malvern Flying Moths of Mothsof Flying fl ying mothsoftheMalvern Hillstoencourage ‘A fullcolourguidetothebutterfl ‘A people toget outrecordingandtoidentify what theyfi nd.’ ies andday Photographs by David Armitage, Bridget Olesky, David Green and Alan Barnes Acknowledgements CONTENTS PAGE Compiled and edited by Susan Clarke and Jenny Joy, helped by many colleagues from Butterfl y Conservation, English Nature, Malvern Hills Introduction 3 AONB Offi ce, Malvern Hills Conservators as well as volunteers, landowners, Management of the Hills 4 The butterfl ies of the Malvern Hills 5 butterfl y and moth recorders, transect walkers and others who provided What are butterfl ies and moths? 5 material, information, advice and commented on the draft. Many thanks to: Why look for butterfl ies and day-fl ying moths? 5 David Armitage, Mike Bradley, Trevor Bucknall, Colin and Helen Dolding, Life cycle 6 Ian Duncan, David Green, Dr Gilbert Greenall, Cherry Greenway, Michael How to identify 7 Harper, Ian Hart, Rob Harvard, Peter Holmes, Chris Johnson, Richard When and where to look 7 Flight periods of butterfl ies and moths 9 Newton, Bridget Oleksy, John Tilt, Trevor Trueman, Gordon Whiting, Mike Sites to visit 10 Williams and Digby Wood. (map centre pages) Recording your sightings 11 Butterfl y research 12 Transect information 12 Species accounts 15 Small Skipper 15 Large Skipper 16 Brimstone 16 Large White, Small White & Green-veined White 16 Orange-tip 17 Green Hairstreak 17 White-letter Hairstreak 18 Small Copper 18 Common Blue 18 Holly Blue 19 Red Admiral, Small Tortoiseshell, Peacock & Comma 19 High Brown Fritillary 20 Silver-washed Fritillary 21 Speckled Wood 21 Marbled White 21 Grayling 22 Small Heath 22 Gatekeeper, Meadow Brown & Ringlet 23 Six-spot Burnet 24 Drab Looper 24 Hummingbird Hawkmoth 24 Scarlet Tiger 25 Cinnabar moth 25 Burnet Companion 25 Important information. -

Gloucestershire Butterflies and Moths - Forest of Dean
Gloucestershire Butterflies and Moths - Forest of Dean Family Name Latin Name Larval Food Plant Flight Characteristics / Other ID Hints Notes Apr July Oct M/B Mar May June Aug Sept Hesperiidae Dingy Skipper B Erynnis tages Bird's-foot Trefoil Uncommon in FoD Hesperiidae Essex Skipper B Thymelicus lineola Grass spp Hard to tell from small skipper Becoming more common. Hesperiidae Grizzled Skipper B Pyrgus malvae Wild Strawberry Uncommon in FoD Hesperiidae Large Skipper B Ochlodes sylvanus Cocksfoot Grass and False Brome Fairly common in FoD Hesperiidae Small Skipper B Thymelius sylvestris Yorkshire Fog, grass spp Fairly common in FoD Lycaenidae Brown Argus B Aricia agestis Rock-rose, Erodium, Geranium Hard to tell from female Common Blue Becoming more common, Lycaenidae Common Blue B Polyommatus icarus Bird's-foot Trefoil, Black Medick, Lesser Trefoil Fairly common in FoD Lycaenidae Holly Blue B Celastrina argiolus H O L L Y I V Y Shrubs - Holly for spring brood, Ivy for summer brood. Population peaks - several year cycle. Fairly common Lycaenidae Purple Hairstreak B Quercusia quercus Oak trees and Oak hedgerows Rarely seen but present in FoD Lycaenidae Small Copper B Lycaena phlaeas Sheep's Sorrel Fairly common Lycaenidae White-letter Hairstreak B Satyrium w-album Larval food plant - Elm. Nectar favourite = Privet + aphid honeydew Rarely seen but present in FoD Nymphalidae Comma B Polygonia c-album Stinging Nettle, Elm, Hop, Bramble Common Nymphalidae Dark Green Fritillary B Argynnis aglaja Hairy Violet, Violet spp Rare in FoD Nymphalidae Painted Lady B Vanessa cardui Thistles Migrant, occasional years is common Nymphalidae Peacock B Inachis io Stinging Nettle Common Nymphalidae Red Admiral B Vanessa atalanta Stinging Nettle. -

Newsletter96.Pdf
The Butterfly Year in Haiku Richard Stewart Under the deep snow And beneath daggers of ice New life is waiting. Late February From a pine's darkness A calm day with warming sun One red admiral seeks sun The first butterfly. And the last nectar. Out of long darkness Juicy chunks of plum A comma with widespread wings A dripping pile on the lawn Soaking up the sun. Feeding butterflies. Prefers hedge garlic Two feeding commas Warmed by rays of evening sun On fermenting blackberries Roosting orange tip. Out of the wind's edge. On such a dull day On grey paving slabs Even a single small white Wide wings of small tortoiseshells Brightens the landscape. Basking in the sun. From a coal blackness Flying to the feast To this large eyed radiance Vanessids land on the first Peacock's open wings. Sunlit buddleia. Along the leaf spine A bright swallowtail Brimstone caterpillar rests Wings luminous in the sun Green on green unseen. Be still and thankful. Green hairstreaks emerge In the bramble glade With a scent of yellow gorse Large whites glide like admirals Heavy on the breeze. Through shafts of sunlight. Two peacocks spiral Up and up into blue sky Drifting with white clouds. It is pleasing that after I wrote that I wanted to see us achieving even more, the Upper Thames branch continues to be increasingly busy and successful, so, thank you to the members who came forward and allowed us to expand our efforts ever further. One of the areas where we could still use some help is in response to requests for us to attend fairs and shows. -

Species Listing Parc Tredelerch
Species Listing Parc Tredelerch Genus /Species Common Name: Alt Name: Type: Arthropod 1 Armadillidium vulgare Pill Woodlouse Type: Bird 45 Acrocephalus scirpaceus Reed Warbler Actitis hypoleucos Common Sandpiper Alauda arvensis Skylark Anas platyrhynchos platyrhynchos Mallard Apus apus Swift Ardea cinerea Grey Heron Heron Aythya fuligula Tufted Duck Buteo Buteo Buzzard Carduelis carduelis Goldfinch Carduelis chloris Greenfinch Cettia cetti Cetti's Warbler Columba palumbus Woodpigeon Corvus corone Carrion Crow Crow Corvus frugilegus Rook Corvus monedula Jackdaw Cygnus olor Mute Swan Delichon urbica House Martin Emberiza schoeniclus Reed Bunting Erithacus rubecula Robin Falco tinnunculus Kestrel Fulica atra Coot Gallinula chloropus Moorhen Hirundo rustica Swallow (barn) Larus argentatus Herring Gull Larus Fuscus Lesser Black Backed Gull Larus ridibundus Black Headed Gull Motacilla alba Pied Wagtail Parus caeruleus Blue Tit Passer domesticus House Sparrow Sparrow Phalacrocorax carbo Cormorant Phylloscopus collybita Chiffchaff Phylloscopus trochilus Willow Warbler Pica pica Magpie Podiceps cristatus Great Crested Grebe Prunella modularis Dunnock Riparia riparia Sand Martin Streptopelia decaocto Collared Dove Sturnus vulgaris Starling Sylvia atricapilla Blackcap Sylvia communis Whitethroat Tadorna tadorna Shell Duck Troglodites troglodites Wren Turdus merula Blackbird Turdus philomelos Song Thrush Vanellus vanellus Lapwing Green Plover Type: Crustacean 1 Gammarus pulex Type: fish 3 Cyprinus carpio Common Carp Gasterosteus aculeatus Three-spined -

Mitcham Common, Entomological Survey 2008
Mitcham Common Entomological survey 2008 Graham A Collins Contents 1. Summary.................................................................................................................................1 2. Methods...................................................................................................................................2 Table 1 – Schedule of survey visits...................................................................................2 3. Results.....................................................................................................................................4 3.1.Species..............................................................................................................................4 Table 2 – Taxonomic summary of the insect groups recorded..........................................4 Table 3 – Rare and Notable species...................................................................................4 Table 4 – UK BAP priority species...................................................................................6 3.2.Compartments..................................................................................................................6 Table 5 - Distribution of species (by National status) across the Compartments..............7 4. Discussion...............................................................................................................................8 4.1.Rare and Notable species.................................................................................................8 -

Butterflies & Moths of the Spanish Pyrenees
Butterflies & Moths of the Spanish Pyrenees Naturetrek Tour Report 5 - 12 July 2017 Mediterranean Burnet Zygaena occitanica by Chris Gibson Spanish Swallowtail by Chris Gibson Owl-fly Libelloides longicornis by Chris Gibson Tibicen plebejus - a large cicada by Chris Gibson Report compiled by Chris Gibson Images courtesy of Neil Holman and Chris Gibson Naturetrek Mingledown Barn Wolf's Lane Chawton Alton Hampshire GU34 3HJ England T: +44 (0)1962 733051 F: +44 (0)1962 736426 E: [email protected] W: www.naturetrek.co.uk Tour Report Butterflies & Moths of the Spanish Pyrenees Tour participants: Chris Gibson & Peter Rich (leaders) together with ten Naturetrek clients Introduction Recent cool and wet weather around Berdún, in the foothills of the Aragónese Pyrenees, had been preceded by several weeks of ferociously hot conditions that threatened to dry out the landscape, shrivel up the nectar sources, and generally bring the butterfly season to a premature close. In the event, we were saved by the most recent weather, and at least when the sun came out the persisting nectar-rich flowers attracted large numbers and a rich diversity of butterflies. We explored from the lowlands to the high mountains in weather that varied from warm and humid to very hot and dry, but with little rain by day at least. In total the week produced 112 species of butterfly, together with many dazzling day-flying moths (particularly burnets) and other wonderful bugs and beasties. Occasional moth trapping gave us a window into the night-life, albeit dominated by Pine Processionary moths, but with a good sample of the big, beautiful and bizarre. -
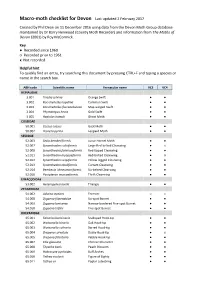
Macro-Moth Checklist for Devon Last Updated 2 February 2017
Macro-moth checklist for Devon Last updated 2 February 2017 Created by Phil Dean on 11 December 2016 using data from the Devon Moth Group database maintained by Dr Barry Henwood (County Moth Recorder) and information from The Moths of Devon (2001) by Roy McCormick. Key ● Recorded since 1960 ○ Recorded prior to 1961 x Not recorded Helpful hint To quickly find an entry, try searching this document by pressing CTRL+F and typing a species or name in the search box. ABH code Scientific name Vernacular name VC3 VC4 HEPIALIDAE 3.001 Triodia sylvina Orange Swift ● ● 3.002 Korscheltellus lupulina Common Swift ● ● 3.003 Korscheltellus fusconebulosa Map-winged Swift ● ● 3.004 Phymatopus hecta Gold Swift ● ● 3.005 Hepialus humuli Ghost Moth ● ● COSSIDAE 50.001 Cossus cossus Goat Moth ● ● 50.002 Zeuzera pyrina Leopard Moth ● ● SESIIDAE 52.003 Sesia bembeciformis Lunar Hornet Moth ● ● 52.007 Synanthedon culiciformis Large Red-belted Clearwing ● x 52.008 Synanthedon formicaeformis Red-tipped Clearwing ● ● 52.011 Synanthedon myopaeformis Red-belted Clearwing ● ○ 52.012 Synanthedon vespiformis Yellow-legged Clearwing ● ● 52.013 Synanthedon tipuliformis Currant Clearwing ● ● 52.014 Bembecia ichneumoniformis Six-belted Clearwing ● ● 52.016 Pyropteron muscaeformis Thrift Clearwing ● ● LIMACODIDAE 53.002 Heterogenea asella Triangle ● ● ZYGAENIDAE 54.002 Adscita statices Forester ○ x 54.008 Zygaena filipendulae Six-spot Burnet ● ● 54.009 Zygaena lonicerae Narrow-bordered Five-spot Burnet ● ● 54.010 Zygaena trifolii Five-spot Burnet ● ● DREPANIDAE 65.001 Falcaria -

Wildlife Travel Vercors 2017
The Vercors, species list and trip report, 26th June to 3rd July 2017 WILDLIFE TRAVEL The Vercors 2017 1 www.wildlife-travel.co.uk The Vercors, species list and trip report, 26th June to 3rd July 2017 # DATE LOCATIONS & NOTES 1 26th June Train to Valence and coach to Les Nonieres 2 27th June Les Nonieres and surrounds 3 28th June Col de Pennes and village of Jansac 4 29th June Les Claps. Marais de Boulignons (Rochbriane), medieval town of Chatillons 5 30th June Cirque d`Archiane 6 1st July Valle de Combeau (lower) 7 2nd July Valle de Combeau (upper), Reserve Naturelle des LIST OF TRAVELLERS Leader Charlie Rugeroni Dorset Graham Bellamy Bedfordshire Cover: Black Vanilla Orchid (Wilf Powell) 2 www.wildlife-travel.co.uk The Vercors, species list and trip report, 26th June to 3rd July 2017 Day 1 Monday 26th June. Outbound from London St Pancras to Valence via Lilles Europe Travellers met prior to boarding the 12.28 Eurostar to Lilles Europe. Two members were temporarily lured by the bright lights and shopping opportunities of Paris but returned for a second look at customs before boarding the correct train. Graham met the group at Lilles before boarding the TGV to Valence where we were met by our driver Serge who drove us the c90 minutes to the hotel Mont Barral where a welcome drink and buffet supper awaited us, before being allocated our rooms by Sylvie. Day 2 Tuesday 27th June. Les Nonieres and surroundings After a brief introduction to the holiday and the areas we would be visiting we set off on foot to explore the area close to the hotel in sunshine.