A Centrality Measure for Cycles and Subgraphs II
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Matroid Theory
MATROID THEORY HAYLEY HILLMAN 1 2 HAYLEY HILLMAN Contents 1. Introduction to Matroids 3 1.1. Basic Graph Theory 3 1.2. Basic Linear Algebra 4 2. Bases 5 2.1. An Example in Linear Algebra 6 2.2. An Example in Graph Theory 6 3. Rank Function 8 3.1. The Rank Function in Graph Theory 9 3.2. The Rank Function in Linear Algebra 11 4. Independent Sets 14 4.1. Independent Sets in Graph Theory 14 4.2. Independent Sets in Linear Algebra 17 5. Cycles 21 5.1. Cycles in Graph Theory 22 5.2. Cycles in Linear Algebra 24 6. Vertex-Edge Incidence Matrix 25 References 27 MATROID THEORY 3 1. Introduction to Matroids A matroid is a structure that generalizes the properties of indepen- dence. Relevant applications are found in graph theory and linear algebra. There are several ways to define a matroid, each relate to the concept of independence. This paper will focus on the the definitions of a matroid in terms of bases, the rank function, independent sets and cycles. Throughout this paper, we observe how both graphs and matrices can be viewed as matroids. Then we translate graph theory to linear algebra, and vice versa, using the language of matroids to facilitate our discussion. Many proofs for the properties of each definition of a matroid have been omitted from this paper, but you may find complete proofs in Oxley[2], Whitney[3], and Wilson[4]. The four definitions of a matroid introduced in this paper are equiv- alent to each other. -

Matroids You Have Known
26 MATHEMATICS MAGAZINE Matroids You Have Known DAVID L. NEEL Seattle University Seattle, Washington 98122 [email protected] NANCY ANN NEUDAUER Pacific University Forest Grove, Oregon 97116 nancy@pacificu.edu Anyone who has worked with matroids has come away with the conviction that matroids are one of the richest and most useful ideas of our day. —Gian Carlo Rota [10] Why matroids? Have you noticed hidden connections between seemingly unrelated mathematical ideas? Strange that finding roots of polynomials can tell us important things about how to solve certain ordinary differential equations, or that computing a determinant would have anything to do with finding solutions to a linear system of equations. But this is one of the charming features of mathematics—that disparate objects share similar traits. Properties like independence appear in many contexts. Do you find independence everywhere you look? In 1933, three Harvard Junior Fellows unified this recurring theme in mathematics by defining a new mathematical object that they dubbed matroid [4]. Matroids are everywhere, if only we knew how to look. What led those junior-fellows to matroids? The same thing that will lead us: Ma- troids arise from shared behaviors of vector spaces and graphs. We explore this natural motivation for the matroid through two examples and consider how properties of in- dependence surface. We first consider the two matroids arising from these examples, and later introduce three more that are probably less familiar. Delving deeper, we can find matroids in arrangements of hyperplanes, configurations of points, and geometric lattices, if your tastes run in that direction. -

Incremental Dynamic Construction of Layered Polytree Networks )
440 Incremental Dynamic Construction of Layered Polytree Networks Keung-Chi N g Tod S. Levitt lET Inc., lET Inc., 14 Research Way, Suite 3 14 Research Way, Suite 3 E. Setauket, NY 11733 E. Setauket , NY 11733 [email protected] [email protected] Abstract Many exact probabilistic inference algorithms1 have been developed and refined. Among the earlier meth ods, the polytree algorithm (Kim and Pearl 1983, Certain classes of problems, including per Pearl 1986) can efficiently update singly connected ceptual data understanding, robotics, discov belief networks (or polytrees) , however, it does not ery, and learning, can be represented as incre work ( without modification) on multiply connected mental, dynamically constructed belief net networks. In a singly connected belief network, there is works. These automatically constructed net at most one path (in the undirected sense) between any works can be dynamically extended and mod pair of nodes; in a multiply connected belief network, ified as evidence of new individuals becomes on the other hand, there is at least one pair of nodes available. The main result of this paper is the that has more than one path between them. Despite incremental extension of the singly connect the fact that the general updating problem for mul ed polytree network in such a way that the tiply connected networks is NP-hard (Cooper 1990), network retains its singly connected polytree many propagation algorithms have been applied suc structure after the changes. The algorithm cessfully to multiply connected networks, especially on is deterministic and is guaranteed to have networks that are sparsely connected. These methods a complexity of single node addition that is include clustering (also known as node aggregation) at most of order proportional to the number (Chang and Fung 1989, Pearl 1988), node elimination of nodes (or size) of the network. -

A Framework for the Evaluation and Management of Network Centrality ∗
A Framework for the Evaluation and Management of Network Centrality ∗ Vatche Ishakiany D´oraErd}osz Evimaria Terzix Azer Bestavros{ Abstract total number of shortest paths that go through it. Ever Network-analysis literature is rich in node-centrality mea- since, researchers have proposed different measures of sures that quantify the centrality of a node as a function centrality, as well as algorithms for computing them [1, of the (shortest) paths of the network that go through it. 2, 4, 10, 11]. The common characteristic of these Existing work focuses on defining instances of such mea- measures is that they quantify a node's centrality by sures and designing algorithms for the specific combinato- computing the number (or the fraction) of (shortest) rial problems that arise for each instance. In this work, we paths that go through that node. For example, in a propose a unifying definition of centrality that subsumes all network where packets propagate through nodes, a node path-counting based centrality definitions: e.g., stress, be- with high centrality is one that \sees" (and potentially tweenness or paths centrality. We also define a generic algo- controls) most of the traffic. rithm for computing this generalized centrality measure for In many applications, the centrality of a single node every node and every group of nodes in the network. Next, is not as important as the centrality of a group of we define two optimization problems: k-Group Central- nodes. For example, in a network of lobbyists, one ity Maximization and k-Edge Centrality Boosting. might want to measure the combined centrality of a In the former, the task is to identify the subset of k nodes particular group of lobbyists. -
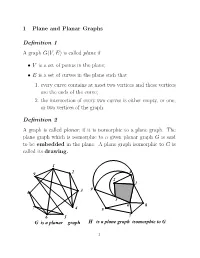
1 Plane and Planar Graphs Definition 1 a Graph G(V,E) Is Called Plane If
1 Plane and Planar Graphs Definition 1 A graph G(V,E) is called plane if • V is a set of points in the plane; • E is a set of curves in the plane such that 1. every curve contains at most two vertices and these vertices are the ends of the curve; 2. the intersection of every two curves is either empty, or one, or two vertices of the graph. Definition 2 A graph is called planar, if it is isomorphic to a plane graph. The plane graph which is isomorphic to a given planar graph G is said to be embedded in the plane. A plane graph isomorphic to G is called its drawing. 1 9 2 4 2 1 9 8 3 3 6 8 7 4 5 6 5 7 G is a planar graph H is a plane graph isomorphic to G 1 The adjacency list of graph F . Is it planar? 1 4 56 8 911 2 9 7 6 103 3 7 11 8 2 4 1 5 9 12 5 1 12 4 6 1 2 8 10 12 7 2 3 9 11 8 1 11 36 10 9 7 4 12 12 10 2 6 8 11 1387 12 9546 What happens if we add edge (1,12)? Or edge (7,4)? 2 Definition 3 A set U in a plane is called open, if for every x ∈ U, all points within some distance r from x belong to U. A region is an open set U which contains polygonal u,v-curve for every two points u,v ∈ U. -

On Hamiltonian Line-Graphsq
ON HAMILTONIANLINE-GRAPHSQ BY GARY CHARTRAND Introduction. The line-graph L(G) of a nonempty graph G is the graph whose point set can be put in one-to-one correspondence with the line set of G in such a way that two points of L(G) are adjacent if and only if the corresponding lines of G are adjacent. In this paper graphs whose line-graphs are eulerian or hamiltonian are investigated and characterizations of these graphs are given. Furthermore, neces- sary and sufficient conditions are presented for iterated line-graphs to be eulerian or hamiltonian. It is shown that for any connected graph G which is not a path, there exists an iterated line-graph of G which is hamiltonian. Some elementary results on line-graphs. In the course of the article, it will be necessary to refer to several basic facts concerning line-graphs. In this section these results are presented. All the proofs are straightforward and are therefore omitted. In addition a few definitions are given. If x is a Une of a graph G joining the points u and v, written x=uv, then we define the degree of x by deg zz+deg v—2. We note that if w ' the point of L(G) which corresponds to the line x, then the degree of w in L(G) equals the degree of x in G. A point or line is called odd or even depending on whether it has odd or even degree. If G is a connected graph having at least one line, then L(G) is also a connected graph. -

Graph Theory to Gain a Deeper Understanding of What’S Going On
Graphs Graphs and trees come up everywhere. I We can view the internet as a graph (in many ways) I who is connected to whom I Web search views web pages as a graph I Who points to whom I Niche graphs (Ecology): I The vertices are species I Two vertices are connected by an edge if they compete (use the same food resources, etc.) Niche graphs describe competitiveness. I Influence Graphs I The vertices are people I There is an edge from a to b if a influences b Influence graphs give a visual representation of power structure. I Social networks: who knows whom There are lots of other examples in all fields . By viewing a situation as a graph, we can apply general results of graph theory to gain a deeper understanding of what's going on. We're going to prove some of those general results. But first we need some notation . Directed and Undirected Graphs A graph G is a pair (V ; E), where V is a set of vertices or nodes and E is a set of edges I We sometimes write G(V ; E) instead of G I We sometimes write V (G) and E(G) to denote the vertices/edges of G. In a directed graph (digraph), the edges are ordered pairs (u; v) 2 V × V : I the edge goes from node u to node v In an undirected simple graphs, the edges are sets fu; vg consisting of two nodes. I there's no direction in the edge from u to v I More general (non-simple) undirected graphs (sometimes called multigraphs) allow self-loops and multiple edges between two nodes. -

5. Matroid Optimization 5.1 Definition of a Matroid
Massachusetts Institute of Technology 18.453: Combinatorial Optimization Michel X. Goemans April 20, 2017 5. Matroid optimization 5.1 Definition of a Matroid Matroids are combinatorial structures that generalize the notion of linear independence in matrices. There are many equivalent definitions of matroids, we will use one that focus on its independent sets. A matroid M is defined on a finite ground set E (or E(M) if we want to emphasize the matroid M) and a collection of subsets of E are said to be independent. The family of independent sets is denoted by I or I(M), and we typically refer to a matroid M by listing its ground set and its family of independent sets: M = (E; I). For M to be a matroid, I must satisfy two main axioms: (I1) if X ⊆ Y and Y 2 I then X 2 I, (I2) if X 2 I and Y 2 I and jY j > jXj then 9e 2 Y n X : X [ feg 2 I. In words, the second axiom says that if X is independent and there exists a larger independent set Y then X can be extended to a larger independent by adding an element of Y nX. Axiom (I2) implies that every maximal (inclusion-wise) independent set is maximum; in other words, all maximal independent sets have the same cardinality. A maximal independent set is called a base of the matroid. Examples. • One trivial example of a matroid M = (E; I) is a uniform matroid in which I = fX ⊆ E : jXj ≤ kg; for a given k. -

6.1 Directed Acyclic Graphs 6.2 Trees
6.1 Directed Acyclic Graphs Directed acyclic graphs, or DAGs are acyclic directed graphs where vertices can be ordered in such at way that no vertex has an edge that points to a vertex earlier in the order. This also implies that we can permute the adjacency matrix in a way that it has only zeros on and below the diagonal. This is a strictly upper triangular matrix. Arranging vertices in such a way is referred to in computer science as topological sorting. Likewise, consider if we performed a strongly connected components (SCC) decomposition, contracted each SCC into a single vertex, and then topologically sorted the graph. If we order vertices within each SCC based on that SCC's order in the contracted graph, the resulting adjacency matrix will have the SCCs in \blocks" along the diagonal. This could be considered a block triangular matrix. 6.2 Trees A graph with no cycle is acyclic.A forest is an acyclic graph. A tree is a connected undirected acyclic graph. If the underlying graph of a DAG is a tree, then the graph is a polytree.A leaf is a vertex of degree one. Every tree with at least two vertices has at least two leaves. A spanning subgraph of G is a subgraph that contains all vertices. A spanning tree is a spanning subgraph that is a tree. All graphs contain at least one spanning tree. Necessary properties of a tree T : 1. T is (minimally) connected 2. T is (maximally) acyclic 3. T has n − 1 edges 4. T has a single u; v-path 8u; v 2 V (T ) 5. -

Introduction to Graph Theory
Introduction to Graph Theory Allen Dickson October 2006 1 The K¨onigsberg Bridge Problem The city of K¨onigsberg was located on the Pregel river in Prussia. The river di- vided the city into four separate landmasses, including the island of Kneiphopf. These four regions were linked by seven bridges as shown in the diagram. Res- idents of the city wondered if it were possible to leave home, cross each of the seven bridges exactly once, and return home. The Swiss mathematician Leon- hard Euler (1707-1783) thought about this problem and the method he used to solve it is considered by many to be the birth of graph theory. x e1 e6 X 6 e2 1 2 e5 wy Y W 5 e4 e3 e7 3 Z 7 4 z Exercise 1.1. See if you can find a round trip through the city crossing each bridge exactly once, or try to explain why such a trip is not possible. The key to Euler’s solution was in a very simple abstraction of the puzzle. Let us redraw our diagram of the city of K¨onigsberg by representing each of the land masses as a vertex and representing each bridge as an edge connecting the vertices corresponding to the land masses. We now have a graph that encodes the necessary information. The problem reduces to finding a ”closed walk” in the graph which traverses each edge exactly once, this is called an Eulerian circuit. Does such a circuit exist? 1 2 Fundamental Definitions We will make the ideas of graphs and circuits from the K¨onigsberg Bridge problem more precise by providing rigorous mathematical definitions. -

Some Trends in Line Graphs
Advances in Theoretical and Applied Mathematics ISSN 0973-4554 Volume 11, Number 2 (2016), pp. 171-178 © Research India Publications http://www.ripublication.com Some Trends In Line Graphs M. Venkatachalapathy, K. Kokila and B. Abarna 1 Department of Mathematics, K.Ramakrishnan College of Engineering, Tiruchirappalli-620 112, Tamil Nadu, India. Abstract In this paper, focus on some trends in line graphs and conclude that we are solving some graphs to satisfied for connected and maximal sub graphs, Further we present a general bounds relating to the line graphs. Introduction In 1736, Euler first introduced the concept of graph theory. In the history of mathematics, the solution given by Euler of the well known Konigsberg bridge problem is considered to be the first theorem of graph theory. This has now become a subject generally regarded as a branch of combinatorics. The theory of graph is an extremely useful tool for solving combinatorial problems in different areas such as geometry, algebra, number theory, topology, operations research, and optimization and computer science. Graphs serve as mathematical models to analyze many concrete real-worldproblems successfully. Certain problems in physics, chemistry, communicationscience, computer technology, genetics, psychology, sociology, and linguistics canbe formulated as problems in graph theory. Also, many branches of mathematics, such as group theory, matrix theory, probability, and topology, have closeconnections with graph theory.Some puzzles and several problems of a practical nature have been instrumentalin the development of various topics in graph theory. The famous K¨onigsbergbridge problem has been the inspiration for the development of Eulerian graphtheory. The challenging Hamiltonian graph theory has been developed from the“Around the World” game of Sir William Hamilton. -

5.6 Euler Paths and Cycles One of the Oldest and Most Beautiful Questions in Graph Theory Originates from a Simple Challenge That Can Be Played by Children
last edited March 16, 2016 5.6 Euler Paths and Cycles One of the oldest and most beautiful questions in graph theory originates from a simple challenge that can be played by children. The town of K¨onigsberg (now Figure 33: An illustration from Euler’s 1741 paper on the subject. Kaliningrad, Russia) is situated near the Pregel River. Residents wondered whether they could they begin a walk in one part of the city and cross each bridge exactly once. Many tried and many failed to find such a path, though understanding why such a path cannot exist eluded them. Graph theory, which studies points and connections between them, is the perfect setting in which to study this question. Land masses can be represented as vertices of a graph, and bridges can be represented as edges between them. Generalizing the question of the K¨onigsberg residents, we might ask whether for a given graph we can “travel” along each of its edges exactly once. Euler’s work elegantly explained why in some graphs such trips are possible and why in some they are not. Definition 23. A path in a graph is a sequence of adjacent edges, such that consecutive edges meet at shared vertices. A path that begins and ends on the same vertex is called a cycle. Note that every cycle is also a path, but that most paths are not cycles. Figure 34 illustrates K5, the complete graph on 5 vertices, with four di↵erent paths highlighted; Figure 35 also illustrates K5, though now all highlighted paths are also cycles.