Final Report (Posted 4/21)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photoionization Studies of Reactive Intermediates Using Synchrotron
Photoionization Studies of Reactive Intermediates using Synchrotron Radiation by John M.Dyke* School of Chemistry University of Southampton SO17 1BJ UK *e-mail: [email protected] 1 Abstract Photoionization of reactive intermediates with synchrotron radiation has reached a sufficiently advanced stage of development that it can now contribute to a number of areas in gas-phase chemistry and physics. These include the detection and spectroscopic study of reactive intermediates produced by bimolecular reactions, photolysis, pyrolysis or discharge sources, and the monitoring of reactive intermediates in situ in environments such as flames. This review summarises advances in the study of reactive intermediates with synchrotron radiation using photoelectron spectroscopy (PES) and constant-ionic-state (CIS) methods with angular resolution, and threshold photoelectron spectroscopy (TPES), taking examples mainly from the recent work of the Southampton group. The aim is to focus on the main information to be obtained from the examples considered. As future research in this area also involves photoelectron-photoion coincidence (PEPICO) and threshold photoelectron-photoion coincidence (TPEPICO) spectroscopy, these methods are also described and previous related work on reactive intermediates with these techniques is summarised. The advantages of using PEPICO and TPEPICO to complement and extend TPES and angularly resolved PES and CIS studies on reactive intermediates are highlighted. 2 1.Introduction This review is organised as follows. After an Introduction to the study of reactive intermediates by photoionization with fixed energy photon sources and synchrotron radiation, a number of Case Studies are presented of the study of reactive intermediates with synchrotron radiation using angle resolved PES and CIS, and TPE spectroscopy. -

SYNTHESIS and PROPERTIES of SOME ARALKYL Hymoperoxides
SYNTHESIS AND PROPERTIES OF SOME ARALKYL HYmOPEROXIDES DISSERTATION Presented in Partial Fulfillment of the Requirements for the Degree Doctor of Philosophy in the Graduate School of The Ohio State University By ARLO d / bCGGS, B.S., M.S. The Ohio State University 1954 Approved by: Department of Chemistry ACKNOWLEDGEMENT The author wishes to express sincere appreciation to Professor Cecil E. Boord for his advice and counsel during this investigation* Thanks also are due Dr. Kenneth W*. Greenlee for his continual interest and guidance and his cooperation in ex tending the facilities of the American Petroleum Institute Research Project 4-5* The financial support of this work by the Firestone Tire and Rubber Company is gratefully acknowledged* ii TABLE OF CONTENTS Page I. INTRODUCTION................................ 1 II. LITERATURE S URVEY ............... 2 III. STATEMENT OF THE PROBLEM.................... 10 IV. DISCUS5IŒ ........................... 11 A. Methods of Preparing Hydroperoxides .... 12 1. Preparation of hydroperoxides from alcohols ............. 12 a. a-methylbenzyl hydroperoxide ...... 12 b. benzyl hydroperoxide .......... l6 c. cinnamyl and a-phenylallyl hydroperoxides 17 d. 1,2,3,4-tetrahydro-l-naphthyl hydro peroxide ............ 22 e. a-indanyl hydroperoxide ........ 23 f. 0-, m- and p-methylbenzyl hydroperoxides 24 g. m- and p-methoxybenzyl hydroperoxides. 28 h. 1,1-diphenylmethyl hydroperoxide .... 31 i. 1,2-diphenylethyl hydroperoxide .... 32 j. 1-a-naphthyl- and l-J3-naphthylethyl hydroperoxides ........ 33 k. 1-styrylethyl hydroperoxide 35 1. 4-a-dimethylbenzyl and 4-methoxy-a- methylbenzyl hydroperoxides ...... 36 m. a-ethylbenzyl and a-ethyl-p-methylbenzyl hydroperoxides ....... ........ 36 n. a-n-propylbenzyl and a-isopropylbenzyl hydroperoxides .................... 37 0. a-2,5“trimethylbenzyl hydroperoxide . -

Fundamentals of Theoretical Organic Chemistry Lecture 9
Fund. Theor. Org. Chem 1 SE Fundamentals of Theoretical Organic Chemistry Lecture 9 1 Fund. Theor. Org. Chem 2 SE 2.2.2 Electrophilic substitution The reaction which takes place between a reactant with an electronegative carbon and an electropositive reagent forming a polarized covalent bond is called electrophilic. In addition, if substitution occurs (i.e. there is a similar polarized covalent bond on the electronegative carbon, which breaks up during the reaction, so the reagent „substitutes” the „old” group or the leaving group) then this specific reaction is called electrophilic substitution. The electronegative carbon is called the reaction centre. In general, the good reactant are molecules having electronegative carbons like aromatic compounds, alkenes and other compounds containing electron-rich double bonds. These are called Lewis bases. On the contrary, good electrophilic reagents are electron poor compounds/molecule groups like acid-halides, which easily form covalent bond with an electronegative centre, thus creating a new molecule. These are often referred to as Lewis acids. According to molecular orbital (MO) theory the driving force for the electrophilic substitution (SE) is a Lewis complex formation involving the LUMO of the Lewis Acid Reagent and the HOMO of the Lewis Base Reactant. Reactant Reagent LUMO LUMO HOMO Lewis base Lewis acid Lewis complex Types of reactions: There are four types of reactions as illustrated below: 2 Fund. Theor. Org. Chem 3 SE Table. 2.2.2-1. Saturated Aromatic SE1 SE1 (Ar) SE2 SE2 (Ar) SE reaction on saturated atom: (1)Unimolecular electrophilic substitution (SE1): The reaction proceeds in two steps. After the departure of the leaving group, the negatively charged reaction intermediate will combine with the reagent. -

One-Pot Syntheses of Irida-Polycyclic Aromatic Hydrocarbons† Cite This: Chem
Chemical Science View Article Online EDGE ARTICLE View Journal | View Issue One-pot syntheses of irida-polycyclic aromatic hydrocarbons† Cite this: Chem. Sci.,2019,10, 10894 a a a b a All publication charges for this article Yu Xuan Hu,‡ Jing Zhang,‡ Xiaoyan Wang, Zhengyu Lu, Fangfang Zhang, have been paid for by the Royal Society Xiaofei Yang,a Zhihua Ma,a Jun Yin, *a Haiping Xia b and Sheng Hua Liu *a of Chemistry Metalla-analogues of polycyclic aromatic hydrocarbons (PAHs) have captivated chemists with their fascinating structures and unique electronic properties. To date, metallabenzene, metallanaphthalene and metallaanthracene have been reported. Metalla-analogues with more complicated fused rings have rarely been reported. Herein, we have successfully synthesized a series of new iridafluoranthenes and fused-ring iridafluoranthenes ranging from pentacyclic to heptacyclic metallaaromatic hydrocarbons in Received 6th August 2019 high yields under mild reaction conditions for the first time. Their photophysical and redox properties Accepted 12th October 2019 were also explored using UV-vis spectroscopy and electrochemistry combined with TD-DFT DOI: 10.1039/c9sc03914g calculations. The present work may offer an important guideline for the design and construction of new rsc.li/chemical-science polycyclic metallaaromatic hydrocarbons and metalla-nanographenes. Creative Commons Attribution-NonCommercial 3.0 Unported Licence. Introduction carbeneiridium compound by using an intramolecular C–H activation reaction.6b In 2018, they further developed irida- Polycyclic aromatic hydrocarbons (PAHs), as important compo- phenanthrene, iridanaphthalene and iridaanthracene from nents in the eld of organic chemistry, have attracted a signi- their corresponding methoxy(alkenyl)carbeneiridium inter- * cant amount of attention due to their wide range of applications mediates via reactions of [IrCp Cl(NCMe) (PMe3)]PF6 with 8 in organic light-emitting diodes,1 eld-effect transistors2 and diarylpropargyl alcohols. -

Bsc Chemistry
Subject Chemistry Paper No and Title 05, ORGANIC CHEMISTRY-II (REACTION MECHANISM-I) Module No and Title 15, The Neighbouring Mechanism, Neighbouring Group Participation by π and σ Bonds Module Tag CHE_P5_M15 CHEMISTRY PAPER :5, ORGANIC CHEMISTRY-II (REACTION MECHANISM-I) MODULE: 15 , The Neighbouring Mechanism, Neighbouring Group Participation by π and σ Bonds TABLE OF CONTENTS 1. Learning Outcomes 2. Introduction 3. NGP Participation 3.1 NGP by Heteroatom Lone Pairs 3.2 NGP by alkene 3.3 NGP by Cyclopropane, Cyclobutane or a Homoallyl group 3.4 NGP by an Aromatic Ring 4. Neighbouring Group Participation on SN2 Reactions 5. Neighbouring Group Participation on SN1 Reactions 6. Neighbouring Group and Rearrangement 7. Examples 8. Summary CHEMISTRY PAPER :5, ORGANIC CHEMISTRY-II (REACTION MECHANISM-I) MODULE: 15 , The Neighbouring Mechanism, Neighbouring Group Participation by π and σ Bonds 1. Learning Outcomes After studying this module, you shall be able to Know about NGP reaction Learn reaction mechanism of NGP reaction Identify stereochemistry of NGP reaction Evaluate the factors affecting the NGP reaction Analyse Phenonium ion, NGP by alkene, and NGP by heteroatom. 2. Introduction The reaction centre (carbenium centre) has direct interaction with a lone pair of electrons of an atom or with the electrons of s- or p-bond present within the parent molecule but these are not in conjugation with the reaction centre. A distinction is sometimes made between n, s and p- participation. An increase in rate due to Neighbouring Group Participation (NGP) is known as "anchimeric assistance". "Synartetic acceleration" happens to be the special case of anchimeric assistance and applies to participation by electrons binding a substituent to a carbon atom in a β- position relative to the leaving group attached to the α-carbon atom. -

Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction
Detection of Phenethylamine, Amphetamine, and Tryptamine Imine By-Products from an Acetone Extraction Mary A. Yohannan* and Arthur Berrier U.S. Department of Justice Drug Enforcement Administration Special Testing and Research Laboratory 22624 Dulles Summit Court Dulles, VA 20166 [email: mary.a.yohannan -at- usdoj.gov] ABSTRACT: The formation of imine by-products from phenethylamines, amphetamines, and tryptamines upon an acetone extraction is presented. These imine by-products were characterized using GC/MSD and exhibited preferential cleavage at the α-carbon of the alkyl chain. Further characterization of the imine by-products of phenethylamine and tryptamine was done using IR and NMR. KEYWORDS: phenethylamine, tryptamine, imine, acetone, schiff base, drug chemistry, forensic chemistry In most forensic laboratories, the solvents used to extract at the α-carbon on the alkyl chain. In addition to GC/MS, the drugs are chosen based upon their solubility properties and their imines formed from phenethylamine base and tryptamine base ability to not interact with the drug. In fact, there are very few were characterized by Fourier transform-infrared spectroscopy publications where a solvent used to extract a drug reacts with (FTIR) and nuclear magnetic resonance (NMR) spectroscopy. the drug and forms by-products [1-3]. This laboratory recently discovered that an additional Experimental component was formed when acetone was used to extract a Solvents, Chemicals, and Materials sample containing a known tryptamine. Analysis by gas Acetone was ACS/HPLC grade from Burdick and Jackson chromatography/mass spectroscopy (GC/MS) of the acetone Laboratories (Muskegon, MI). Phenethylamine base and extract yielded an extra peak in the total ion chromatogram that tryptamine base were obtained from Sigma-Aldrich Chemicals was approximately half the abundance of the known tryptamine (Milwaukee, WI). -

Linking Chemical Reaction Intermediates of the Click Reaction to Their Molecular Diffusivity
1 Linking chemical reaction intermediates of the click reaction to their molecular diffusivity Tian Huang,a Bo Li,a Huan Wang,b* and Steve Granicka,c* a Center for Soft and Living Matter, Institute for Basic Science (IBS), Ulsan 44919, South Korea b College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, P. R. China c Departments of Chemistry and Physics, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, South Korea Abstract Bipolar reactions have been provoked by reports of boosted diffusion during chemical and enzymatic reactions. To some, it is intuitively reasonable that relaxation to truly Brownian motion after passing an activation barrier can be slow, but to others the notion is so intuitively unphysical that they suspect the supporting experiments to be artifact. Here we study a chemical reaction according to whose mechanism some intermediate species should speed up while others slow down in predictable ways, if the boosted diffusion interpretation holds. Experimental artifacts would do not know organic chemistry mechanism, however. Accordingly, we scrutinize the absolute diffusion coefficient (D) during intermediate stages of the CuAAC reaction (copper- catalyzed azide-alkyne cycloaddition click reaction), using proton pulsed field-gradient nuclear magnetic resonance (PFG-NMR) to discriminate between the diffusion of various reaction intermediates. For the azide reactant, its D increases during reaction, peaks at the same time as peak reaction rate, then returns to its initial value. For the alkyne reagent, its D decreases consistent with presence of the intermediate large complexes formed from copper catalyst and its ligand, except for the 2Cu-alk complex whose more rapid D may signify that this species is the 2 real reactive complex. -

Gas Phase Ion/Molecule Reactions As Studied by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry
INIS-mf—10165 GAS PHASE ION/MOLECULE REACTIONS AS STUDIED BY FOURIER TRANSFORM ION CYCLOTRON RESONANCE MASS SPECTROMETRY STEEN INGEMANN J0RGENSEN GAS PHASE ION/MOLECULE REACTIONS AS STUDIED BY FOURIER TRANSFORM ION CYCLOTRON RESONANCE MASS SPECTROMETRY ACADEMISCH PROEFSCHRIFT ter verkrijging van de graad van doctor in de Wiskunde en Natuurwetenschappen aan de Universiteit van Amsterdam, op gezag van de Rector Magnificus dr. D.W. Bresters, hoogleraar in de Faculteit der Wiskunde en Natuurwetenschappen, in het openbaar te verdedigen in de Aula der Universiteit (tijdelijk in het Wiskundegebouw, Roetersstraat 15) op woensdag 12 juni 1985 te 16.00 uur. door STEEN INGEMANN J0RGENSEN geboren te Kopenhagen 1985 Offsetdrukkerij Kanters B.V., Alblasserdam PROMOTOR: Prof. Dr. N.M.M. Nibbering Part of the work described in this thesis has been accomplished under the auspices of the Netherlands Foundation for Chemical Research (SON) with the financial support from the Netherlands Organization for the Advancement of Pure Research (ZWO). STELLINGEN 1. De door White e.a. getrokken conclusie, dat het cycloheptatrieen anion omlegt tot het benzyl anion in de gasfase, is onvoldoende ondersteund door de experimentele gegevens. R.L. White, CL. Wilkins, J.J. Heitkamp, S.W. Staley, J. Am. Chem. Soc, _105, 4868 (1983). 2. De gegeven experimentele methode voor de lithiëring van endo- en exo- -5-norborneen-2,3-dicarboximide is niet in overeenstemming met de ge- postuleerde vorming van een algemeen dianion van deze verbindingen. P.J. Garratt, F. Hollowood, J. Org. Chem., 47, 68 (1982). 3. De door Barton e.a. gegeven verklaring voor de observaties, dat O-alkyl- -S-alkyl-dithiocarbonaten reageren met N^-dimethylhydrazine onder vor- ming van ^-alkyl-thiocarbamaten en ^-alkyl-thiocarbazaten, terwijl al- koxythiocarbonylimidazolen uitsluitend £-alkyl-thiocarbazaten geven, is hoogst twijfelachtig. -

Use Stable Isotopes to Investigate Microbial H2 and N2o Production
USE STABLE ISOTOPES TO INVESTIGATE MICROBIAL H2 AND N2O PRODUCTION By Hui Yang A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Biochemistry and Molecular Biology - Doctor of Philosophy 2015 ABSTRACT USE STABLE ISOTOPES TO INVESTIGATE MICROBIAL H2 AND N2O PRODUCTION By Hui Yang Stable isotopes can be a useful tool in studying the basic processes involved in enzymatic catalysis. Isotope effects are quantifiable values related to the substitution of isotopes. It derives from the difference in zero-point energies. In contrast to non-catalyzed reactions, enzyme- catalyzed reactions involve multiple steps, the overall isotope effects are the sum of the isotope effects in each step. There are two major kinds of isotope effects, equilibrium isotope effects (EIEs) and kinetic isotope effects (KIEs). Each of them can provide us insights into different states in the reaction. This thesis describes several researches related to using the stable isotopes to study microbial metabolism. It is demonstrated in Chapter 2 that a method is developed for the measurement of H isotope fractionation patterns in hydrogenases. After the development of the method, a detailed study of the H2 metabolism catalyzed by different hydrogenases is presented in Chapter 3. The methods developed in hydrogenase studies were deployed in nitric oxide reductase-catalyzed N2O production studies, which is described in Chapter 4. TABLE OF CONTENTS LIST OF TABLES………………………………………………………………………………..vi LIST OF FIGURES……………………………………………………………………………...vii -
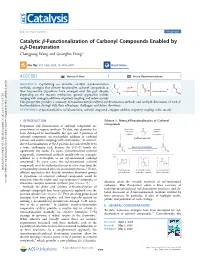
Catalytic Β-Functionalization of Carbonyl Compounds Enabled by Α
pubs.acs.org/acscatalysis Perspective Catalytic β‑Functionalization of Carbonyl Compounds Enabled by α,β-Desaturation Chengpeng Wang and Guangbin Dong* Cite This: ACS Catal. 2020, 10, 6058−6070 Read Online ACCESS Metrics & More Article Recommendations ABSTRACT: Capitalizing on versatile catalytic α,β-desaturation methods, strategies that directly functionalize carbonyl compounds at their less-reactive β-positions have emerged over the past decade. Depending on the reaction mechanism, general approaches include merging with conjugate addition, migratory coupling, and redox cascade. This perspective provides a summary of transition-metal-catalyzed α,β-desaturation methods and in-depth discussions of each β- functionalization strategy with their advantages, challenges, and future directions. KEYWORDS: β-functionalization, α,β-desaturation, carbonyl compound, conjugate addition, migratory coupling, redox cascade I. INTRODUCTION Scheme 1. Direct β-Functionalization of Carbonyl Compounds Preparation and derivatization of carbonyl compounds are cornerstones in organic synthesis. To date, rich chemistry has been developed to functionalize the ipso and α-positions of carbonyl compounds via nucleophilic addition to carbonyl carbons and enolate couplings with electrophiles.1 In contrast, direct functionalization at the β position has undoubtedly been a more challenging task, because the β-C−H bonds are significantly less acidic. To access β-functionalized carbonyl compounds, conventional methods mainly rely on conjugate addition of a nucleophile to an α,β-unsaturated carbonyl compound.2 In many cases, the α,β-unsaturated carbonyl compounds must be synthesized in one or a few steps from the 3 Downloaded via UNIV OF CHICAGO on May 19, 2021 at 13:46:31 (UTC). corresponding saturated ones via an oxidation process. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

The Synthesis and Characterisation of a New Tröger's Base Content
Sys Rev Pharm 2020;11(7):319-323 A multifaceted review journal in the field of pharmacy The Synthesis and Characterisation of a New Tröger’s Base Content Methoxy Group Sadiq A. Karim1, Mohammed H. Said2, Jinan A. Abd3, Asim A. Balakit4, Ayad F. Alkaim5 1,2,5Chemistry department, College of Science for women, University of Babylon, Iraq. 3Department of Laser physics, College of Science for women, University of Babylon, Iraq. 4Pharmaceutical Chemistry department, College of Pharmacy, University of Babylon, Iraq. Corresponding author: [email protected] ABSTRACT Five Tröger’s base (TB) molecules were synthesized by reaction aniline’s Keywords: Tröger base, heterocyclic compounds, chirality derivatives which content a methoxy group with a supplement of methylene (dimethoxymethane (DMM)) in present trifluoroacetic acid (TFA) as a solvent Correspondence: and catalyst. This method afforded a good ratio product between 62% to 99%, Sadiq A. Karim1 all products were conforming by FTIR, HRMs, 1HNMR, 13CNMR, and XRD. 1,2,5Chemistry department, College of Science for women, University of Babylon, Iraq. Corresponding author: [email protected] INTRODUCTION collected by filtration and was dried in a vacuum oven at The first Tröger’s base was create in 1887 by Julius Tröger 50 °C for 2 h. during his Ph.D. studied, this compound was called Tröger base 1 (TB1) which from the condensation of methanal 1- Synthesis of 2,9-Dimethoxy-6H,12H-5,11- with 4-aminotoluene in HCl-catalysed media.1 The TB1 methanodibenzo[b,f](1,5)diazocine (TB-OCH3-1) (2,8-dimethyl-6H,12H-5,11- General procedure was followed using m-anisidine (9.1 methanodibenzo[b,f][1,5]diazocine) was conformed ml, 10.00 g, 81.20 mmol), DMM (10.8 ml, 9.269 g, 121.80 structure through chemical reaction by M.