Bsc Chemistry
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Photoionization Studies of Reactive Intermediates Using Synchrotron
Photoionization Studies of Reactive Intermediates using Synchrotron Radiation by John M.Dyke* School of Chemistry University of Southampton SO17 1BJ UK *e-mail: [email protected] 1 Abstract Photoionization of reactive intermediates with synchrotron radiation has reached a sufficiently advanced stage of development that it can now contribute to a number of areas in gas-phase chemistry and physics. These include the detection and spectroscopic study of reactive intermediates produced by bimolecular reactions, photolysis, pyrolysis or discharge sources, and the monitoring of reactive intermediates in situ in environments such as flames. This review summarises advances in the study of reactive intermediates with synchrotron radiation using photoelectron spectroscopy (PES) and constant-ionic-state (CIS) methods with angular resolution, and threshold photoelectron spectroscopy (TPES), taking examples mainly from the recent work of the Southampton group. The aim is to focus on the main information to be obtained from the examples considered. As future research in this area also involves photoelectron-photoion coincidence (PEPICO) and threshold photoelectron-photoion coincidence (TPEPICO) spectroscopy, these methods are also described and previous related work on reactive intermediates with these techniques is summarised. The advantages of using PEPICO and TPEPICO to complement and extend TPES and angularly resolved PES and CIS studies on reactive intermediates are highlighted. 2 1.Introduction This review is organised as follows. After an Introduction to the study of reactive intermediates by photoionization with fixed energy photon sources and synchrotron radiation, a number of Case Studies are presented of the study of reactive intermediates with synchrotron radiation using angle resolved PES and CIS, and TPE spectroscopy. -

Fundamentals of Theoretical Organic Chemistry Lecture 9
Fund. Theor. Org. Chem 1 SE Fundamentals of Theoretical Organic Chemistry Lecture 9 1 Fund. Theor. Org. Chem 2 SE 2.2.2 Electrophilic substitution The reaction which takes place between a reactant with an electronegative carbon and an electropositive reagent forming a polarized covalent bond is called electrophilic. In addition, if substitution occurs (i.e. there is a similar polarized covalent bond on the electronegative carbon, which breaks up during the reaction, so the reagent „substitutes” the „old” group or the leaving group) then this specific reaction is called electrophilic substitution. The electronegative carbon is called the reaction centre. In general, the good reactant are molecules having electronegative carbons like aromatic compounds, alkenes and other compounds containing electron-rich double bonds. These are called Lewis bases. On the contrary, good electrophilic reagents are electron poor compounds/molecule groups like acid-halides, which easily form covalent bond with an electronegative centre, thus creating a new molecule. These are often referred to as Lewis acids. According to molecular orbital (MO) theory the driving force for the electrophilic substitution (SE) is a Lewis complex formation involving the LUMO of the Lewis Acid Reagent and the HOMO of the Lewis Base Reactant. Reactant Reagent LUMO LUMO HOMO Lewis base Lewis acid Lewis complex Types of reactions: There are four types of reactions as illustrated below: 2 Fund. Theor. Org. Chem 3 SE Table. 2.2.2-1. Saturated Aromatic SE1 SE1 (Ar) SE2 SE2 (Ar) SE reaction on saturated atom: (1)Unimolecular electrophilic substitution (SE1): The reaction proceeds in two steps. After the departure of the leaving group, the negatively charged reaction intermediate will combine with the reagent. -

Reaction Kinetics in Organic Reactions
Autumn 2004 Reaction Kinetics in Organic Reactions Why are kinetic analyses important? • Consider two classic examples in asymmetric catalysis: geraniol epoxidation 5-10% Ti(O-i-C3H7)4 O DET OH * * OH + TBHP CH2Cl2 3A mol sieve OH COOH5C2 L-(+)-DET = OH COOH5C2 * OH geraniol hydrogenation OH 0.1% Ru(II)-BINAP + H2 CH3OH P(C6H5)2 (S)-BINAP = P(C6 H5)2 • In both cases, high enantioselectivities may be achieved. However, there are fundamental differences between these two reactions which kinetics can inform us about. 1 Autumn 2004 Kinetics of Asymmetric Catalytic Reactions geraniol epoxidation: • enantioselectivity is controlled primarily by the preferred mode of initial binding of the prochiral substrate and, therefore, the relative stability of intermediate species. The transition state resembles the intermediate species. Finn and Sharpless in Asymmetric Synthesis, Morrison, J.D., ed., Academic Press: New York, 1986, v. 5, p. 247. geraniol hydrogenation: • enantioselectivity may be dictated by the relative reactivity rather than the stability of the intermediate species. The transition state may not resemble the intermediate species. for example, hydrogenation of enamides using Rh+(dipamp) studied by Landis and Halpern (JACS, 1987, 109,1746) 2 Autumn 2004 Kinetics of Asymmetric Catalytic Reactions “Asymmetric catalysis is four-dimensional chemistry. Simple stereochemical scrutiny of the substrate or reagent is not enough. The high efficiency that these reactions provide can only be achieved through a combination of both an ideal three-dimensional structure (x,y,z) and suitable kinetics (t).” R. Noyori, Asymmetric Catalysis in Organic Synthesis,Wiley-Interscience: New York, 1994, p.3. “Studying the photograph of a racehorse cannot tell you how fast it can run.” J. -

Linking Chemical Reaction Intermediates of the Click Reaction to Their Molecular Diffusivity
1 Linking chemical reaction intermediates of the click reaction to their molecular diffusivity Tian Huang,a Bo Li,a Huan Wang,b* and Steve Granicka,c* a Center for Soft and Living Matter, Institute for Basic Science (IBS), Ulsan 44919, South Korea b College of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, P. R. China c Departments of Chemistry and Physics, Ulsan National Institute of Science and Technology (UNIST), Ulsan 44919, South Korea Abstract Bipolar reactions have been provoked by reports of boosted diffusion during chemical and enzymatic reactions. To some, it is intuitively reasonable that relaxation to truly Brownian motion after passing an activation barrier can be slow, but to others the notion is so intuitively unphysical that they suspect the supporting experiments to be artifact. Here we study a chemical reaction according to whose mechanism some intermediate species should speed up while others slow down in predictable ways, if the boosted diffusion interpretation holds. Experimental artifacts would do not know organic chemistry mechanism, however. Accordingly, we scrutinize the absolute diffusion coefficient (D) during intermediate stages of the CuAAC reaction (copper- catalyzed azide-alkyne cycloaddition click reaction), using proton pulsed field-gradient nuclear magnetic resonance (PFG-NMR) to discriminate between the diffusion of various reaction intermediates. For the azide reactant, its D increases during reaction, peaks at the same time as peak reaction rate, then returns to its initial value. For the alkyne reagent, its D decreases consistent with presence of the intermediate large complexes formed from copper catalyst and its ligand, except for the 2Cu-alk complex whose more rapid D may signify that this species is the 2 real reactive complex. -

Use Stable Isotopes to Investigate Microbial H2 and N2o Production
USE STABLE ISOTOPES TO INVESTIGATE MICROBIAL H2 AND N2O PRODUCTION By Hui Yang A DISSERTATION Submitted to Michigan State University in partial fulfillment of the requirements for the degree of Biochemistry and Molecular Biology - Doctor of Philosophy 2015 ABSTRACT USE STABLE ISOTOPES TO INVESTIGATE MICROBIAL H2 AND N2O PRODUCTION By Hui Yang Stable isotopes can be a useful tool in studying the basic processes involved in enzymatic catalysis. Isotope effects are quantifiable values related to the substitution of isotopes. It derives from the difference in zero-point energies. In contrast to non-catalyzed reactions, enzyme- catalyzed reactions involve multiple steps, the overall isotope effects are the sum of the isotope effects in each step. There are two major kinds of isotope effects, equilibrium isotope effects (EIEs) and kinetic isotope effects (KIEs). Each of them can provide us insights into different states in the reaction. This thesis describes several researches related to using the stable isotopes to study microbial metabolism. It is demonstrated in Chapter 2 that a method is developed for the measurement of H isotope fractionation patterns in hydrogenases. After the development of the method, a detailed study of the H2 metabolism catalyzed by different hydrogenases is presented in Chapter 3. The methods developed in hydrogenase studies were deployed in nitric oxide reductase-catalyzed N2O production studies, which is described in Chapter 4. TABLE OF CONTENTS LIST OF TABLES………………………………………………………………………………..vi LIST OF FIGURES……………………………………………………………………………...vii -

S.T.E.T.Women's College, Mannargudi Semester Iii Ii M
S.T.E.T.WOMEN’S COLLEGE, MANNARGUDI SEMESTER III II M.Sc., CHEMISTRY ORGANIC CHEMISTRY - II – P16CH31 UNIT I Aliphatic nucleophilic substitution – mechanisms – SN1, SN2, SNi – ion-pair in SN1 mechanisms – neighbouring group participation, non-classical carbocations – substitutions at allylic and vinylic carbons. Reactivity – effect of structure, nucleophile, leaving group and stereochemical factors – correlation of structure with reactivity – solvent effects – rearrangements involving carbocations – Wagner-Meerwein and dienone-phenol rearrangements. Aromatic nucleophilic substitutions – SN1, SNAr, Benzyne mechanism – reactivity orientation – Ullmann, Sandmeyer and Chichibabin reaction – rearrangements involving nucleophilic substitution – Stevens – Sommelet Hauser and von-Richter rearrangements. NUCLEOPHILIC SUBSTITUTION Mechanism of Aliphatic Nucleophilic Substitution. Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. For that reason, it attracts to nucleophile In organic chemistry and inorganic chemistry, nucleophilic substitution is a fundamental class of reactions in which a leaving group(nucleophile) is replaced by an electron rich compound(nucleophile). The whole molecular entity of which the electrophile and the leaving group are part is usually called the substrate. The nucleophile essentially attempts to replace the leaving group as the primary substituent in the reaction itself, as a part of another molecule. The most general form of the reaction may be given as the following: Nuc: + R-LG → R-Nuc + LG: The electron pair (:) from the nucleophile(Nuc) attacks the substrate (R-LG) forming a new 1 bond, while the leaving group (LG) departs with an electron pair. The principal product in this case is R-Nuc. The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. -
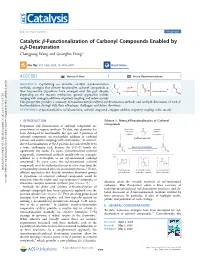
Catalytic Β-Functionalization of Carbonyl Compounds Enabled by Α
pubs.acs.org/acscatalysis Perspective Catalytic β‑Functionalization of Carbonyl Compounds Enabled by α,β-Desaturation Chengpeng Wang and Guangbin Dong* Cite This: ACS Catal. 2020, 10, 6058−6070 Read Online ACCESS Metrics & More Article Recommendations ABSTRACT: Capitalizing on versatile catalytic α,β-desaturation methods, strategies that directly functionalize carbonyl compounds at their less-reactive β-positions have emerged over the past decade. Depending on the reaction mechanism, general approaches include merging with conjugate addition, migratory coupling, and redox cascade. This perspective provides a summary of transition-metal-catalyzed α,β-desaturation methods and in-depth discussions of each β- functionalization strategy with their advantages, challenges, and future directions. KEYWORDS: β-functionalization, α,β-desaturation, carbonyl compound, conjugate addition, migratory coupling, redox cascade I. INTRODUCTION Scheme 1. Direct β-Functionalization of Carbonyl Compounds Preparation and derivatization of carbonyl compounds are cornerstones in organic synthesis. To date, rich chemistry has been developed to functionalize the ipso and α-positions of carbonyl compounds via nucleophilic addition to carbonyl carbons and enolate couplings with electrophiles.1 In contrast, direct functionalization at the β position has undoubtedly been a more challenging task, because the β-C−H bonds are significantly less acidic. To access β-functionalized carbonyl compounds, conventional methods mainly rely on conjugate addition of a nucleophile to an α,β-unsaturated carbonyl compound.2 In many cases, the α,β-unsaturated carbonyl compounds must be synthesized in one or a few steps from the 3 Downloaded via UNIV OF CHICAGO on May 19, 2021 at 13:46:31 (UTC). corresponding saturated ones via an oxidation process. -

Reactions of Alkenes and Alkynes
05 Reactions of Alkenes and Alkynes Polyethylene is the most widely used plastic, making up items such as packing foam, plastic bottles, and plastic utensils (top: © Jon Larson/iStockphoto; middle: GNL Media/Digital Vision/Getty Images, Inc.; bottom: © Lakhesis/iStockphoto). Inset: A model of ethylene. KEY QUESTIONS 5.1 What Are the Characteristic Reactions of Alkenes? 5.8 How Can Alkynes Be Reduced to Alkenes and 5.2 What Is a Reaction Mechanism? Alkanes? 5.3 What Are the Mechanisms of Electrophilic Additions HOW TO to Alkenes? 5.1 How to Draw Mechanisms 5.4 What Are Carbocation Rearrangements? 5.5 What Is Hydroboration–Oxidation of an Alkene? CHEMICAL CONNECTIONS 5.6 How Can an Alkene Be Reduced to an Alkane? 5A Catalytic Cracking and the Importance of Alkenes 5.7 How Can an Acetylide Anion Be Used to Create a New Carbon–Carbon Bond? IN THIS CHAPTER, we begin our systematic study of organic reactions and their mecha- nisms. Reaction mechanisms are step-by-step descriptions of how reactions proceed and are one of the most important unifying concepts in organic chemistry. We use the reactions of alkenes as the vehicle to introduce this concept. 129 130 CHAPTER 5 Reactions of Alkenes and Alkynes 5.1 What Are the Characteristic Reactions of Alkenes? The most characteristic reaction of alkenes is addition to the carbon–carbon double bond in such a way that the pi bond is broken and, in its place, sigma bonds are formed to two new atoms or groups of atoms. Several examples of reactions at the carbon–carbon double bond are shown in Table 5.1, along with the descriptive name(s) associated with each. -

CHEMICAL KINETICS Pt 2 Reaction Mechanisms Reaction Mechanism
Reaction Mechanism (continued) CHEMICAL The reaction KINETICS 2 C H O +→5O + 6CO 4H O Pt 2 3 4 3 2 2 2 • has many steps in the reaction mechanism. Objectives ! Be able to describe the collision and Reaction Mechanisms transition-state theories • Even though a balanced chemical equation ! Be able to use the Arrhenius theory to may give the ultimate result of a reaction, determine the activation energy for a reaction and to predict rate constants what actually happens in the reaction may take place in several steps. ! Be able to relate the molecularity of the reaction and the reaction rate and • This “pathway” the reaction takes is referred to describle the concept of the “rate- as the reaction mechanism. determining” step • The individual steps in the larger overall reaction are referred to as elementary ! Be able to describe the role of a catalyst and homogeneous, heterogeneous and reactions. enzyme catalysis Reaction Mechanisms Often Used Terms •Intermediate: formed in one step and used up in a subsequent step and so is never seen as a product. The series of steps by which a chemical reaction occurs. •Molecularity: the number of species that must collide to produce the reaction indicated by that A chemical equation does not tell us how step. reactants become products - it is a summary of the overall process. •Elementary Step: A reaction whose rate law can be written from its molecularity. •uni, bi and termolecular 1 Elementary Reactions Elementary Reactions • Consider the reaction of nitrogen dioxide with • Each step is a singular molecular event carbon monoxide. -

SN2 , SN1 , E2 , & E1: Substitution and Elimination Reactions
SN2 , SN1 , E2 , & E1: Substitution and Elimination Reactions • Nucleophilic Substitution Reactions (SN2 and SN1) replace a leaving group with a nucleophile (Nu: or Nu: - ) • Elimination Reactions (E2 and E1) generate a double bond by loss of " A+ " and " B: - " • They may compete with each other Nucleophilic Substitution Reactions - SN2 Reaction: • Reaction is: o Stereospecific (Walden Inversion of configuration) o Concerted - all bonds form and break at same time o Bimolecular - rate depends on concentration of both nucleophile and substrate • Substrate: o Best if primary (one substituent on carbon bearing leaving group) o works if secondary, fails if tertiary • Nucleophile: o Best if more reactive (i.e. more anionic or more basic) • Leaving Group: Best if more stable (i.e. can support negative charge well): o TsO- (very good) > I- > Br- > Cl- > F- (poor) o RF , ROH , ROR , RNH2 are NEVER Substrates for SN2 reactions o Leaving Groups on double-bonded carbons are never replaced by SN2 reactions • Solvent: Polar Aprotic (i.e. no OH) is best. o For example dimethylsulfoxide ( CH3SOCH3 ), dimethylformamide ( HCON(CH3)2 ), acetonitrile ( CH3CN ). o Protic solvents (e.g. H2O or ROH) deactivate nucleophile by hydrogen bonding but can be used in some case Intro Chem Handouts Substitution & Elimination Reactions Page 1 of 3 Nucleophilic Substitution Reactions – SN1 Reaction: • Reaction is: o Non-stereospecific (attack by nucleophile occurs from both sides) o Non-concerted - has carbocation intermediate o Unimolecular - rate depends on concentration of only the substrate • Substrate: o Best if tertiary or conjugated (benzylic or allylic) carbocation can be formed as leaving group departs o never primary • Nucleophile: o Best if more reactive (i.e. -

Elimination Reactions Are Described
Introduction In this module, different types of elimination reactions are described. From a practical standpoint, elimination reactions widely used for the generation of double and triple bonds in compounds from a saturated precursor molecule. The presence of a good leaving group is a prerequisite in most elimination reactions. Traditional classification of elimination reactions, in terms of the molecularity of the reaction is employed. How the changes in the nature of the substrate as well as reaction conditions affect the mechanism of elimination are subsequently discussed. The stereochemical requirements for elimination in a given substrate and its consequence in the product stereochemistry is emphasized. ELIMINATION REACTIONS Objective and Outline beta-eliminations E1, E2 and E1cB mechanisms Stereochemical considerations of these reactions Examples of E1, E2 and E1cB reactions Alpha eliminations and generation of carbene I. Basics Elimination reactions involve the loss of fragments or groups from a molecule to generate multiple bonds. A generalized equation is shown below for 1,2-elimination wherein the X and Y from two adjacent carbon atoms are removed, elimination C C C C -XY X Y Three major types of elimination reactions are: α-elimination: two atoms or groups are removed from the same atom. It is also known as 1,1-elimination. H R R C X C + HX R Both H and X are removed from carbon atom here R Carbene β-elimination: loss of atoms or groups on adjacent atoms. It is also H H known as 1,2- elimination. R C C R R HC CH R X H γ-elimination: loss of atoms or groups from the 1st and 3rd positions as shown below. -

Oct 16 2018 Chem 261 Notes
CHEM 261 October 9, 2018 RECALL: Identification of Chiral and Achiral (not chiral) compounds Example: Diaminopimelic acid - The above molecule is achiral even though there are stereogenic center (s), because there is symmetry within the molecule - These kinds of molecules are called meso compounds, which are compounds that contain stereocenters yet because of their symmetry, have mirror images that can be superimposed. - All achiral molecules, including meso compounds do not rotate polarized light (i.e. [α]D = 0) - Diaminopimelic acid - a component of bacterial cell wall and biosynthetic precursor to the amino acid known as lysine - this R,S diaminopimelic acid (above) is a diastereomer of the enantiomers (S,S or R,R diaminopimelic acid) below: Mirror O O O O C S C C R C HO S OH HO R OH NH2 NH2 NH2 NH2 chiral chiral Enantiomers A racemic mixture (racemate) of two enantiomers in a 1:1 ratio also has an [α]D = 0 Resolution of Enantiomers Definition: separation of two enantiomers - Requires a chiral reagent The starting material lactic acids are enantiomers of each other. By reacting enantiomers to make a salt with an enantiomer of MDMA (another chiral molecule), one can obtain salts which are now diastereomers of each other (RS and RR). The resulting diastereomers have different melting points, boiling points, solubilities, and can be separated by crystallization. >>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>> Substitution Reactions Remember: Radical Substitution light R-H + X2 R-X + H-X Proceeds by a radical mechanism Alkane X=F, Cl, Br Alkyl Halide Not I Ionic Substitution: M Nu: + R-X Nu-R + :X M Nucleophile Alkyl Halide (Seeks positive charge) Nucleophile is a subtsnace that seeks positive charge Types of Nucleophilic Substitution (SN) SN1 - rate depends on 1 concentration SN2 - The rate is dependent on the concentration of the nucleophile and the nucleophile (2 concentrations) Sn2 Mechanism Reverse reaction will not occur.