The Morphology of Xenarthrous Vertebrae (Mammalia: Xenarthra)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The World at the Time of Messel: Conference Volume
T. Lehmann & S.F.K. Schaal (eds) The World at the Time of Messel - Conference Volume Time at the The World The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment and the History of Early Primates 22nd International Senckenberg Conference 2011 Frankfurt am Main, 15th - 19th November 2011 ISBN 978-3-929907-86-5 Conference Volume SENCKENBERG Gesellschaft für Naturforschung THOMAS LEHMANN & STEPHAN F.K. SCHAAL (eds) The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference Frankfurt am Main, 15th – 19th November 2011 Conference Volume Senckenberg Gesellschaft für Naturforschung IMPRINT The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates 22nd International Senckenberg Conference 15th – 19th November 2011, Frankfurt am Main, Germany Conference Volume Publisher PROF. DR. DR. H.C. VOLKER MOSBRUGGER Senckenberg Gesellschaft für Naturforschung Senckenberganlage 25, 60325 Frankfurt am Main, Germany Editors DR. THOMAS LEHMANN & DR. STEPHAN F.K. SCHAAL Senckenberg Research Institute and Natural History Museum Frankfurt Senckenberganlage 25, 60325 Frankfurt am Main, Germany [email protected]; [email protected] Language editors JOSEPH E.B. HOGAN & DR. KRISTER T. SMITH Layout JULIANE EBERHARDT & ANIKA VOGEL Cover Illustration EVELINE JUNQUEIRA Print Rhein-Main-Geschäftsdrucke, Hofheim-Wallau, Germany Citation LEHMANN, T. & SCHAAL, S.F.K. (eds) (2011). The World at the Time of Messel: Puzzles in Palaeobiology, Palaeoenvironment, and the History of Early Primates. 22nd International Senckenberg Conference. 15th – 19th November 2011, Frankfurt am Main. Conference Volume. Senckenberg Gesellschaft für Naturforschung, Frankfurt am Main. pp. 203. -

SMC 136 Gazin 1958 1 1-112.Pdf
SMITHSONIAN MISCELLANEOUS COLLECTIONS VOLUME 136, NUMBER 1 Cftarlesi 3B, anb JKarp "^aux OTalcott 3^es(earcf) Jf unb A REVIEW OF THE MIDDLE AND UPPER EOCENE PRIMATES OF NORTH AMERICA (With 14 Plates) By C. LEWIS GAZIN Curator, Division of Vertebrate Paleontology United States National Museum Smithsonian Institution (Publication 4340) CITY OF WASHINGTON PUBLISHED BY THE SMITHSONIAN INSTITUTION JULY 7, 1958 THE LORD BALTIMORE PRESS, INC. BALTIMORE, MD., U. S. A. CONTENTS Page Introduction i Acknowledgments 2 History of investigation 4 Geographic and geologic occurrence 14 Environment I7 Revision of certain lower Eocene primates and description of three new upper Wasatchian genera 24 Classification of middle and upper Eocene forms 30 Systematic revision of middle and upper Eocene primates 31 Notharctidae 31 Comparison of the skulls of Notharctus and Smilodectcs z:^ Omomyidae 47 Anaptomorphidae 7Z Apatemyidae 86 Summary of relationships of North American fossil primates 91 Discussion of platyrrhine relationships 98 References 100 Explanation of plates 108 ILLUSTRATIONS Plates (All plates follow page 112) 1. Notharctus and Smilodectes from the Bridger middle Eocene. 2. Notharctus and Smilodectes from the Bridger middle Eocene. 3. Notharctus and Smilodectcs from the Bridger middle Eocene. 4. Notharctus and Hemiacodon from the Bridger middle Eocene. 5. Notharctus and Smilodectcs from the Bridger middle Eocene. 6. Omomys from the middle and lower Eocene. 7. Omomys from the middle and lower Eocene. 8. Hemiacodon from the Bridger middle Eocene. 9. Washakius from the Bridger middle Eocene. 10. Anaptomorphus and Uintanius from the Bridger middle Eocene. 11. Trogolemur, Uintasorex, and Apatcmys from the Bridger middle Eocene. 12. Apatemys from the Bridger middle Eocene. -

71St Annual Meeting Society of Vertebrate Paleontology Paris Las Vegas Las Vegas, Nevada, USA November 2 – 5, 2011 SESSION CONCURRENT SESSION CONCURRENT
ISSN 1937-2809 online Journal of Supplement to the November 2011 Vertebrate Paleontology Vertebrate Society of Vertebrate Paleontology Society of Vertebrate 71st Annual Meeting Paleontology Society of Vertebrate Las Vegas Paris Nevada, USA Las Vegas, November 2 – 5, 2011 Program and Abstracts Society of Vertebrate Paleontology 71st Annual Meeting Program and Abstracts COMMITTEE MEETING ROOM POSTER SESSION/ CONCURRENT CONCURRENT SESSION EXHIBITS SESSION COMMITTEE MEETING ROOMS AUCTION EVENT REGISTRATION, CONCURRENT MERCHANDISE SESSION LOUNGE, EDUCATION & OUTREACH SPEAKER READY COMMITTEE MEETING POSTER SESSION ROOM ROOM SOCIETY OF VERTEBRATE PALEONTOLOGY ABSTRACTS OF PAPERS SEVENTY-FIRST ANNUAL MEETING PARIS LAS VEGAS HOTEL LAS VEGAS, NV, USA NOVEMBER 2–5, 2011 HOST COMMITTEE Stephen Rowland, Co-Chair; Aubrey Bonde, Co-Chair; Joshua Bonde; David Elliott; Lee Hall; Jerry Harris; Andrew Milner; Eric Roberts EXECUTIVE COMMITTEE Philip Currie, President; Blaire Van Valkenburgh, Past President; Catherine Forster, Vice President; Christopher Bell, Secretary; Ted Vlamis, Treasurer; Julia Clarke, Member at Large; Kristina Curry Rogers, Member at Large; Lars Werdelin, Member at Large SYMPOSIUM CONVENORS Roger B.J. Benson, Richard J. Butler, Nadia B. Fröbisch, Hans C.E. Larsson, Mark A. Loewen, Philip D. Mannion, Jim I. Mead, Eric M. Roberts, Scott D. Sampson, Eric D. Scott, Kathleen Springer PROGRAM COMMITTEE Jonathan Bloch, Co-Chair; Anjali Goswami, Co-Chair; Jason Anderson; Paul Barrett; Brian Beatty; Kerin Claeson; Kristina Curry Rogers; Ted Daeschler; David Evans; David Fox; Nadia B. Fröbisch; Christian Kammerer; Johannes Müller; Emily Rayfield; William Sanders; Bruce Shockey; Mary Silcox; Michelle Stocker; Rebecca Terry November 2011—PROGRAM AND ABSTRACTS 1 Members and Friends of the Society of Vertebrate Paleontology, The Host Committee cordially welcomes you to the 71st Annual Meeting of the Society of Vertebrate Paleontology in Las Vegas. -

A Pangolin Genome Hub for the Research Community
Database, 2016, 1–10 doi: 10.1093/database/baw063 Original article Original article PGD: a pangolin genome hub for the research community Tze King Tan1,2, Ka Yun Tan1,3, Ranjeev Hari1,2, Aini Mohamed Yusoff1,2, Guat Jah Wong1, Cheuk Chuen Siow1, Naresh V.R. Mutha1, Mike Rayko4, Aleksey Komissarov4, Pavel Dobrynin4, Ksenia Krasheninnikova4, Gaik Tamazian4, Ian C. Paterson2,5, Wesley C. Warren6, Warren E. Johnson7, Stephen J. O’Brien4,8 and Siew Woh Choo1,2,9,* 1Genome Informatics Research Laboratory, Centre for Research in Biotechnology for Agriculture (CEBAR), High Impact Research Building, University of Malaya, 50603 Kuala Lumpur, Malaysia, 2Department of Oral and Craniofacial Sciences, Faculty of Dentistry, University of Malaya, 50603 Kuala Lumpur, Malaysia, 3Institute of Biology Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur Malaysia, 4Theodosius Dobzhansky Center for Genome Bioinformatics, Saint Petersburg State University, St. Petersburg 199004, Russia, 5Oral Cancer Research and Coordinating Centre, Faculty of Dentistry, University of Malaya, 50603 Kuala Lumpur, Malaysia, 6McDonnell Genome Institute, Washington University, St Louis, MO 63108, USA, 7Smithsonian Conservation Biology Institute, Front Royal, Virginia 22630, USA, 8Oceanographic Center, Nova Southeastern University, Ft Lauderdale, FL, 33004, USA and 9Genome Solutions Sdn Bhd, Suite 8, Innovation Incubator UM, Level 5, Research Management & Innovation Complex, University of Malaya, 50603 Kuala Lumpur, Malaysia *Corresponding author: Tel: þ603-79676463; Email: [email protected] Citation details: Tan,T.K., Tan,K.Y., Hari,R. et al. PGD: a pangolin genome hub for the research community. Database (2016) Vol. 2016: article ID baw063; doi:doi:10.1093/database/baw063 Received 24 October 2015; Revised 15 March 2016; Accepted 11 April 2016 Abstract Pangolins (order Pholidota) are the only mammals covered by scales. -

Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming
Distribution and Stratigraphip Correlation of Upper:UB_ • Ju Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Iktman Formations, Southern Bighorn Basin* Wyoming U,S. GEOLOGICAL SURVEY PROFESS IONAL PAPER 1540 Cover. A member of the American Museum of Natural History 1896 expedition enter ing the badlands of the Willwood Formation on Dorsey Creek, Wyoming, near what is now U.S. Geological Survey fossil vertebrate locality D1691 (Wardel Reservoir quadran gle). View to the southwest. Photograph by Walter Granger, courtesy of the Department of Library Services, American Museum of Natural History, New York, negative no. 35957. DISTRIBUTION AND STRATIGRAPHIC CORRELATION OF UPPER PALEOCENE AND LOWER EOCENE FOSSIL MAMMAL AND PLANT LOCALITIES OF THE FORT UNION, WILLWOOD, AND TATMAN FORMATIONS, SOUTHERN BIGHORN BASIN, WYOMING Upper part of the Will wood Formation on East Ridge, Middle Fork of Fifteenmile Creek, southern Bighorn Basin, Wyoming. The Kirwin intrusive complex of the Absaroka Range is in the background. View to the west. Distribution and Stratigraphic Correlation of Upper Paleocene and Lower Eocene Fossil Mammal and Plant Localities of the Fort Union, Willwood, and Tatman Formations, Southern Bighorn Basin, Wyoming By Thomas M. Down, Kenneth D. Rose, Elwyn L. Simons, and Scott L. Wing U.S. GEOLOGICAL SURVEY PROFESSIONAL PAPER 1540 UNITED STATES GOVERNMENT PRINTING OFFICE, WASHINGTON : 1994 U.S. DEPARTMENT OF THE INTERIOR BRUCE BABBITT, Secretary U.S. GEOLOGICAL SURVEY Robert M. Hirsch, Acting Director For sale by U.S. Geological Survey, Map Distribution Box 25286, MS 306, Federal Center Denver, CO 80225 Any use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. -

Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field
Attachment J Assessment of Existing Paleontologic Data Along with Field Survey Results for the Jonah Field June 12, 2007 ABSTRACT This is compilation of a technical analysis of existing paleontological data and a limited, selective paleontological field survey of the geologic bedrock formations that will be impacted on Federal lands by construction associated with energy development in the Jonah Field, Sublette County, Wyoming. The field survey was done on approximately 20% of the field, primarily where good bedrock was exposed or where there were existing, debris piles from recent construction. Some potentially rich areas were inaccessible due to biological restrictions. Heavily vegetated areas were not examined. All locality data are compiled in the separate confidential appendix D. Uinta Paleontological Associates Inc. was contracted to do this work through EnCana Oil & Gas Inc. In addition BP and Ultra Resources are partners in this project as they also have holdings in the Jonah Field. For this project, we reviewed a variety of geologic maps for the area (approximately 47 sections); none of maps have a scale better than 1:100,000. The Wyoming 1:500,000 geology map (Love and Christiansen, 1985) reveals two Eocene geologic formations with four members mapped within or near the Jonah Field (Wasatch – Alkali Creek and Main Body; Green River – Laney and Wilkins Peak members). In addition, Winterfeld’s 1997 paleontology report for the proposed Jonah Field II Project was reviewed carefully. After considerable review of the literature and museum data, it became obvious that the portion of the mapped Alkali Creek Member in the Jonah Field is probably misinterpreted. -

Influence of Tertiary Paleoenvironmental Changes On
BMC Evolutionary Biology BioMed Central Research article Open Access Influence of Tertiary paleoenvironmental changes on the diversification of South American mammals: a relaxed molecular clock study within xenarthrans Frédéric Delsuc*1,2, Sergio F Vizcaíno3 and Emmanuel JP Douzery1 Address: 1Laboratoire de Paléontologie, Paléobiologie et Phylogénie, Institut des Sciences de l'Evolution, Université Montpellier II, Montpellier, France, 2The Allan Wilson Centre for Molecular Ecology and Evolution, Massey University, Palmerston North, New Zealand and 3Departamento Científico Paleontología de Vertebrados, Museo de La Plata, Paseo del Bosque s/n, 1900 La Plata, Argentina Email: Frédéric Delsuc* - [email protected]; Sergio F Vizcaíno - [email protected]; Emmanuel JP Douzery - [email protected] * Corresponding author Published: 28 April 2004 Received: 25 December 2003 Accepted: 28 April 2004 BMC Evolutionary Biology 2004, 4:11 This article is available from: http://www.biomedcentral.com/1471-2148/4/11 © 2004 Delsuc et al; licensee BioMed Central Ltd. This is an Open Access article: verbatim copying and redistribution of this article are permitted in all media for any purpose, provided this notice is preserved along with the article's original URL. MammalsXenarthransEvolutionPalaeontologyPhylogenyRelaxed molecular clockBayesian datingGlobal changeTertiarySouth America Abstract Background: Comparative genomic data among organisms allow the reconstruction of their phylogenies and evolutionary time scales. Molecular -

Resolving the Relationships of Paleocene Placental Mammals
Biol. Rev. (2015), pp. 000–000. 1 doi: 10.1111/brv.12242 Resolving the relationships of Paleocene placental mammals Thomas J. D. Halliday1,2,∗, Paul Upchurch1 and Anjali Goswami1,2 1Department of Earth Sciences, University College London, Gower Street, London WC1E 6BT, U.K. 2Department of Genetics, Evolution and Environment, University College London, Gower Street, London WC1E 6BT, U.K. ABSTRACT The ‘Age of Mammals’ began in the Paleocene epoch, the 10 million year interval immediately following the Cretaceous–Palaeogene mass extinction. The apparently rapid shift in mammalian ecomorphs from small, largely insectivorous forms to many small-to-large-bodied, diverse taxa has driven a hypothesis that the end-Cretaceous heralded an adaptive radiation in placental mammal evolution. However, the affinities of most Paleocene mammals have remained unresolved, despite significant advances in understanding the relationships of the extant orders, hindering efforts to reconstruct robustly the origin and early evolution of placental mammals. Here we present the largest cladistic analysis of Paleocene placentals to date, from a data matrix including 177 taxa (130 of which are Palaeogene) and 680 morphological characters. We improve the resolution of the relationships of several enigmatic Paleocene clades, including families of ‘condylarths’. Protungulatum is resolved as a stem eutherian, meaning that no crown-placental mammal unambiguously pre-dates the Cretaceous–Palaeogene boundary. Our results support an Atlantogenata–Boreoeutheria split at the root of crown Placentalia, the presence of phenacodontids as closest relatives of Perissodactyla, the validity of Euungulata, and the placement of Arctocyonidae close to Carnivora. Periptychidae and Pantodonta are resolved as sister taxa, Leptictida and Cimolestidae are found to be stem eutherians, and Hyopsodontidae is highly polyphyletic. -

Rapid and Early Post-Flood Mammalian Diversification Videncede in the Green River Formation
The Proceedings of the International Conference on Creationism Volume 6 Print Reference: Pages 449-457 Article 36 2008 Rapid and Early Post-Flood Mammalian Diversification videncedE in the Green River Formation John H. Whitmore Cedarville University Kurt P. Wise Southern Baptist Theological Seminary Follow this and additional works at: https://digitalcommons.cedarville.edu/icc_proceedings DigitalCommons@Cedarville provides a publication platform for fully open access journals, which means that all articles are available on the Internet to all users immediately upon publication. However, the opinions and sentiments expressed by the authors of articles published in our journals do not necessarily indicate the endorsement or reflect the views of DigitalCommons@Cedarville, the Centennial Library, or Cedarville University and its employees. The authors are solely responsible for the content of their work. Please address questions to [email protected]. Browse the contents of this volume of The Proceedings of the International Conference on Creationism. Recommended Citation Whitmore, John H. and Wise, Kurt P. (2008) "Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation," The Proceedings of the International Conference on Creationism: Vol. 6 , Article 36. Available at: https://digitalcommons.cedarville.edu/icc_proceedings/vol6/iss1/36 In A. A. Snelling (Ed.) (2008). Proceedings of the Sixth International Conference on Creationism (pp. 449–457). Pittsburgh, PA: Creation Science Fellowship and Dallas, TX: Institute for Creation Research. Rapid and Early Post-Flood Mammalian Diversification Evidenced in the Green River Formation John H. Whitmore, Ph.D., Cedarville University, 251 N. Main Street, Cedarville, OH 45314 Kurt P. Wise, Ph.D., Southern Baptist Theological Seminary, 2825 Lexington Road. -

Citogenética Molecular Y Reproducción De La Tribu Euphractini Winge, 1923 (Dasypodidae, Xenarthra, Mammalia) De Argentina
Tesis Doctoral Citogenética molecular y reproducción de la tribu Euphractini Winge, 1923 (Dasypodidae, Xenarthra, Mammalia) de Argentina Luaces, Juan Pablo 2011-12-12 Este documento forma parte de la colección de tesis doctorales y de maestría de la Biblioteca Central Dr. Luis Federico Leloir, disponible en digital.bl.fcen.uba.ar. Su utilización debe ser acompañada por la cita bibliográfica con reconocimiento de la fuente. This document is part of the doctoral theses collection of the Central Library Dr. Luis Federico Leloir, available in digital.bl.fcen.uba.ar. It should be used accompanied by the corresponding citation acknowledging the source. Cita tipo APA: Luaces, Juan Pablo. (2011-12-12). Citogenética molecular y reproducción de la tribu Euphractini Winge, 1923 (Dasypodidae, Xenarthra, Mammalia) de Argentina. Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. Cita tipo Chicago: Luaces, Juan Pablo. "Citogenética molecular y reproducción de la tribu Euphractini Winge, 1923 (Dasypodidae, Xenarthra, Mammalia) de Argentina". Facultad de Ciencias Exactas y Naturales. Universidad de Buenos Aires. 2011-12-12. Dirección: Biblioteca Central Dr. Luis F. Leloir, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires. Contacto: [email protected] Intendente Güiraldes 2160 - C1428EGA - Tel. (++54 +11) 4789-9293 UNIVERSIDAD DE BUENOS AIRES FACULTAD DE CIENCIAS EXACTAS Y NATURALES Citogenética molecular y reproducción de la tribu Euphractini Winge, 1923 (Dasypodidae, Xenarthra, Mammalia) de Argentina. Tesis presentada para optar al título de Doctor de la Universidad de Buenos Aires en el área: CIENCIAS BIOLÓGICAS Lic. Juan Pablo Luaces Director: Prof. Dra. María Susana Merani Consejero de estudios: Prof. Dra. -

Ankle Structure in Eocene Pholidotan Mammal Eomanis Krebsi and Its Taxonomic Implications
Ankle structure in Eocene pholidotan mammal Eomanis krebsi and its taxonomic implications Inés Horovitz, Gerhard Storch, and Thomas Martin Acta Palaeontologica Polonica 50 (3), 2005: 545-548 Georges Cuvier (1798) established the classical concept of Edentata which included sloths, anteaters, armadillos, aardvarks, and pangolins. With the growing body of comparative morphological data becoming available during the nineteenth century, it was evident that Cuvier's “Edentata” was an artificial group (e.g., Huxley 1872). In his classical textbook,Weber (1904) excluded aardvarks and pangolins from the Edentata and put them in separate orders, Tubulidentata and Pholidota. Later on, fossil taxa were repeatedly added to and removed from Edentata, such as various xenarthran groups, taeniodonts, palaeanodonts, and gondwanatheres, but the South American Xenarthra always was considered as their core group. Even the living order Pholidota has been cited again as ?Edentata incertae sedis many years after Weber’s work (Romer 1966). The validity and extent of a higher taxon Edentata are still in dispute. In this discussion, the Middle Eocene pholidotan Eomanis and the putative xenarthran Eurotamandua from Grube Messel near Darmstadt (Germany) play an important role (Storch 1978, 1981, 2003; Rose and Emry 1993; Gaudin and Branham 1998; Rose 1999). Eomanis krebsi and Eurotamandua joresi have been subject to some discussion regarding their taxonomic distinction. It has been suggested that the only specimen known of Eo. krebsi might actually be a juvenile representative of the senior species E. joresi. A reexamination of the type specimen of Eo. krebsi has yielded some new observations regarding the identity of some of its ankle elements. -
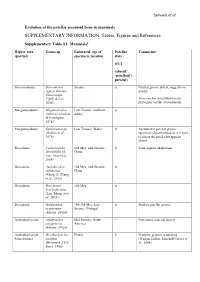
SUPPLEMENTARY INFORMATION: Tables, Figures and References
Samuels et al. Evolution of the patellar sesamoid bone in mammals SUPPLEMENTARY INFORMATION: Tables, Figures and References Supplementary Table S1: Mammals$ Higher taxa Genus sp. Estimated. age of Patellar Comments# (partial) specimen, location state 0/1/2 (absent/ ‘patelloid’/ present) Sinoconodonta Sinoconodon Jurassic 0 Patellar groove absent, suggests no rigneyi (Kielan- patella Jaworowska, Cifelli & Luo, Sinoconodon is included on our 2004) phylogeny within tritylodontids. Morganucodonta Megazostrodon Late Triassic, southern 0 rudnerae (Jenkins Africa & Parrington, 1976) Morganucodonta Eozostrodon sp. Late Triassic, Wales 0 Asymmetric patellar groove, (Jenkins et al., specimens disarticulated so it is hard 1976) to assess the patella but appears absent Docodonta Castorocauda 164 Mya, mid-Jurassic, 0 Semi-aquatic adaptations lutrasimilis (Ji, China Luo, Yuan et al., 2006) Docodonta Agilodocodon 164 Mya, mid-Jurassic, 0 scansorius China (Meng, Ji, Zhang et al., 2015) Docodonta Docofossor 160 Mya 0 brachydactylus (Luo, Meng, Ji et al., 2015) Docodonta Haldanodon 150-155 Mya, Late 0 Shallow patellar groove exspectatus Jurassic, Portugal (Martin, 2005b) Australosphenida Asfaltomylos Mid-Jurassic, South ? Postcranial material absent patagonicus America (Martin, 2005a) Australosphenida Ornithorhynchus Extant 2 Platypus, genome sequenced Monotremata anatinus (Warren, Hillier, Marshall Graves et (Herzmark, 1938; al., 2008) Rowe, 1988) Samuels et al. Australosphenida Tachyglossus + Extant 2 Echidnas Monotremata Zaglossus spp. (Herzmark, 1938; Rowe, 1988) Mammaliaformes Fruitafossor 150 Mya, Late Jurassic, 0 Phylogenetic status uncertain indet. windscheffeli (Luo Colorado & Wible, 2005) Mammaliaformes Volaticotherium Late Jurassic/Early ? Hindlimb material incomplete indet. antiquus (Meng, Cretaceous Hu, Wang et al., 2006) Eutriconodonta Jeholodens 120-125 Mya, Early 0 Poorly developed patellar groove jenkinsi (Ji, Luo Cretaceous, China & Ji, 1999) Eutriconodonta Gobiconodon spp.